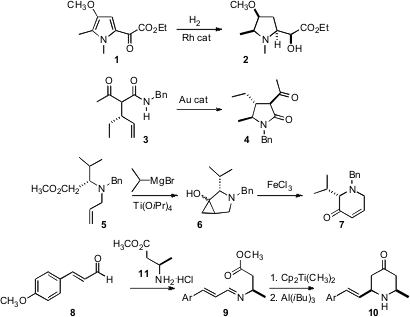
Several methods have been reported for the stereocontrolled preparation of pyrrolidine and piperidine derivatives. Alison J. Frontier of the University of Rochester has observed (Org. Lett. 2007, 9, 4939.DOI: 10.1021/ol701962w)that hydrogenation of acyl pyrroles such as 1 gave good control not just around the ring, but also on the sidechain. Chi-Ming Che of the University of Hong Kong has devised (J. Am. Chem. Soc. 2007, 129, 5828.DOI: 10.1021/ja070027j)a catalyst that converted amides such as 3 into the cyclized product 4, also with high diastereocontrol. Jean Ollivier of the Université de Paris-Sud, following the Sato procedure, has applied (Tetrahedron Lett. 2007, 48, 8765.DOI: 10.1016/j.tetlet.2007.10.003)the Kulinkovich reaction to allylated amino acid esters such as 5, to give, after Fe-mediated fragmentation, the enantiomerically-pure piperidone 7. Richard C. PMID:24059181 BuyMal-amido-PEG8-NHS ester Hartley of the University of Glasgow has reported (J. Org. 3-Chloro-5-nitro-1H-pyrazole site Chem. 2007, 72, 10287. DOI: 10.1021/jo7019948)what are, remarkably, the first examples of the aza-Petasis-Ferrier reaction, converting an ester such as 9, by carbonyl methylenation followed byMannich cyclization, into the piperidone 10.
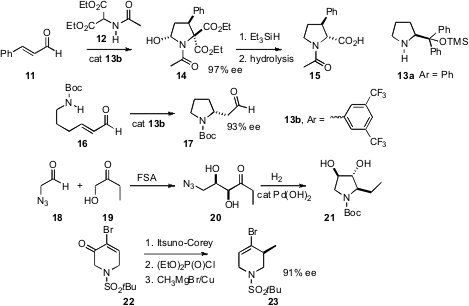
Procedures for catalytic enantioselective C-N ring construction have also been developed. Armando Córdova of Stockholm University has shown (Tetrahedron Lett. 2007, 48, 8695.DOI: 10.1016/j.tetlet.2007.10.028)that condensation of 11 with 12 led to 14, which on reduction and hydrolysis delivered the 3-aryl proline 15. In an even simpler case, Santos Fustero of the Universidad de Valencia found (Org. Lett. 2007, 9, 5283.DOI: 10.1021/ol702447y)that the aldehyde 16 could cyclize to 17 with high ee. In a different approach (J. Am. Chem. Soc. 2007, 129, 14811.DOI: 10.1021/ja073911i), William E. Greenberg and Chi-Huey Wong of Scripps/La Jolla harnessed the power of an enzyme to mediate the addition of 19 to 18, leading to the pyrrolidine 21. Daniel P. Furkert of the University of Bath has applied (Org. Lett. 2007, 9, 3769.DOI: 10.1021/ol0713988)the powerful Itsuno-Corey reduction to the piperidone 22, leading, after SN2′ displacement, to the alkene23.
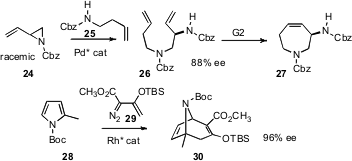
To construct larger rings, Barry M. Trost of Stanford University has employed (Angew. Chem. Int. Ed. 2007, 46, 6123.DOI: 10.1002/anie.200700835)his powerful Pd catalyst to effect opening of the racemic aziridine 24, leading, after metathesis, to the amine 27. Huw M. L. Davies of the University at Buffalo used the Rh catalyst he has devised (J. Am. Chem. Soc. 2007, 129, 10312.DOI: 10.1021/ja072936e)to mediate the addition of 29 to the pyrrole 28, giving the bicyclic 30 in high ee.