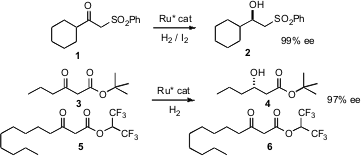
Secondary alcohols can be prepared in high enantiomeric excess by catalytic hydrogenation of ketones. Zhaoguo Zhang of Shanghai Jiaotong University has established (Org. Lett. 2007, 9, 5613. DOI: 10.1021/ol702565x)that β-keto sulfones such as 1 are suitable substrates for this hydrogenation. 1-Chloro-6-iodohexane Price Reinhard Brückner of the Universität Freiburg has demonstrated (Angew. Chem. Int. Ed. 2007, 46, 6537. DOI: 10.1002/anie.200700021)that the rate of hydrogenation of β-keto esters such as 3 and 5 depends on the alcohol from which the ester is derived, so 3 can be reduced to 4 in the presence of 5. PMID:23695992
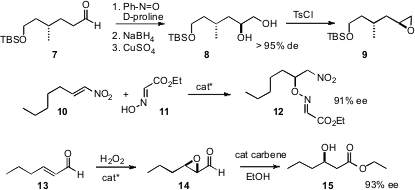
Enantiomerically-pure secondary alcohols and amines can also be prepared by adding an oxygen or a nitrogen to an existing carbon skeleton. Both Srivari Chandrasekhar of the Indian Institute of Chemical Technology, Hyderabad (Tetrahedron Lett. 2007, 48, 7339. DOI: 10.1016/j.tetlet.2007.08.017)and Arumugam Sudalai of the National Chemical Laboratory, Pune (Tetrahedron Lett. 2007, 48, 8544. 3-Sulfopropanoic acid site DOI: 10.1016/j.tetlet.2007.09.133)have taken advantage of the previously-described enantioselective α-aminoxylation of aldehydes to establish what appears to be a robust preparative route to the enantiomerically-pureepoxides such as 9 of terminal alkenes. Karl Anker Jørgensen of Aarhus University has developed (Chem. Commun. 2007, 3646.DOI: 10.1039/b707844g)a catalyst for the enantioselective addition of 11 to nitroalkenes such as 10. Armando Córdova of Stockholm University has shown (Tetrahedron Lett. 2007, 48, 5976.DOI: 10.1016/j.tetlet.2007.06.110)that epoxy aldehydes such as 14, easily prepared by the protocol he developed, are converted by the Bode catalyst to β-hydroxy esters such as 15.
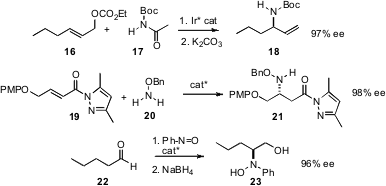
Hyunsoo Han of the University of Texas, San Antonio has described (Tetrahedron Lett. 2007, 48, 7094.DOI: 10.1016/j.tetlet.2007.08.009)an improved protocol for the enantioselective conversion of primary allylic carbonates 16 to secondary amines 17. René Peters of ETH Zurich has used (Angew. Chem. Int. Ed. 2007, 46, 7704.DOI: 10.1002/anie.200702086)a related procedure for the construction of aminated quaternary centers. Mukund P. Sibi of North Dakota State University has devised (J. Am. Chem. Soc. 2007, 129, 8064.DOI: 10.1021/ja071739c)a catalyst for the conjugate addition of the benzyloxyamine 20 to acyl pyrazoles, and Claudio Palomo of the Universidad de País Vasco has found (Angew. Chem. Int. Ed. 2007, 46, 8054.DOI: 10.1002/anie.200703001)that a simple diphenyl prolinol catalyst will effect enantioselective α-amination of aldehydes.
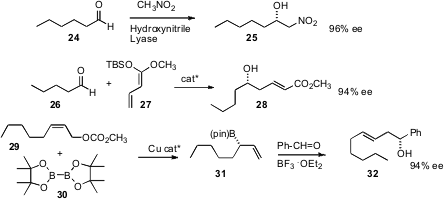
Carbon-carbon bond formation can also be used to assemble enantiomerically-pure secondary alcohols. Herfried Griengl of Graz University of Technology has found (Adv. Synth. Catal. 2007, 349, 1445.DOI: 10.1002/adsc.200700064)that a commercial nitrile lyase effects addition of nitromethane to an aldehyde such as 24 to give the nitro alcohol 25 in high ee. Markus Kalesse of Leibniz Universität Hannover has constructed a catalyst (Org. Lett. 2007, 9, 5637.DOI: 10.1021/ol702640w)for the enantioselective addition of the ketene silyl acetal 27 to aldehydes. Hajime Ito and Masaya Sawamura of Hokkaido University (J. Am. Chem. Soc. 2007, 129, 14856. DOI: 10.1021/ja076634o)(depicted), and Dennis G. Hall of the University of Alberta (Angew. Chem. Int. Ed. 2007, 46, 5913.DOI: 10.1002/anie.200700975)have reported complementary enantioselective preparations of allyl boronates such as 31.