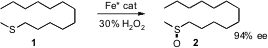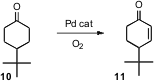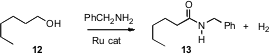Although the enantioselective oxidation of alkyl aryl sulfides is well
developed, much less is known about dialkyl sulfides. Tsutomu Katsuki of Kyushu
University has designed (J. Am. Chem. Soc. PMID:26446225 2007, 129, 8940.
DOI: 10.1021/ja071916+)
an
Fe(salan) complex that combines with aqueous
H2O2 to
oxidize alkyl methyl sulfides in high ee.
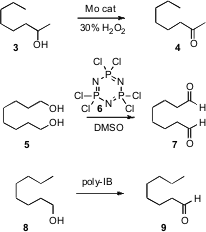
The oxidation of alcohols to aldehydes and ketones is one of the most widely
practiced of synthetic transformations. 2,2′:6′,2”-Terpyridine structure Ge Wang of the University of Science and
Technology in Beijing has developed (Chem. Formula of 312624-65-0 Lett. 2007, 36,
1236.
DOI: 10.1246/cl.2007.1236)
a Mo catalyst that used aqueous H2O2 to effect this
transformation. Secondary alcohols are oxidized more rapidly than primary
alcohols. Vinod K. Singh of the Indian Institute of Technology, Kanpur, has
found (Synth. Comm. 2007, 37, 4099.
DOI: 10.1080/00397910701572597)
that the solid, inexpensive 6 can take the place of oxalyl chloride in
the Swern oxidation. Viktor V. Zhdankin of the University of Minnesota, Duluth
has devised (J. Org. Chem. 2007, 72, 8149.
DOI: 10.1021/jo7015746)
a
polymer-bound
hypervalent iodine reagent that is easily separated after use, and
reoxidized for reuse.
Enones such as 11 are versatile intermediates for organic synthesis.
Makoto Tokunaga, now at Kyushu University, and Yasushi Tsuji, now at Kyoto
University, have found (Tetrahedron Lett. 2007, 48, 6860.
DOI: 10.1016/j.tetlet.2007.07.181)
a Pd catalyst that, in the presence of
O2, will oxidize a cyclic
ketone such as 10 to the enone.
The direct oxidation of an alcohol to the acid is not always an efficient
process, so the conversion of 12 to 13 would often be carried out
over at least three steps. David Milstein of the Weizmann Institute of Science
has devised (Science 2007, 317, 790.
DOI: 10.1126/science.1145295)
a
Ru catalyst that effected the transformation in a single step, generating H2
as a byproduct as the oxidation proceeded.
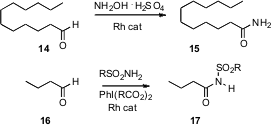
The oxidation of an aldehyde to the corresponding amide is also a useful
transformation. Noritaka Mizuno of the University of Tokyo has designed (Angew.
Chem. Int. Ed. 2007, 46, 5202.
DOI: 10.1002/anie.200701273)
a Rh
catalyst that can combine, in water, the aldehyde 14 and NH2OH
to give the primary amide 15. Johann Chan of Amgen Inc., Thousand Oaks,
CA has found (J. Am. Chem. Soc. 2007, 129, 14106.
DOI: 10.1021/ja073872a)
a
different Rh catalyst that mediated the oxidation of a sulfonamide to the
nitrene, which under the reaction conditions inserted into the aldehyde H to
give the amide 17.
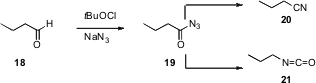
Krishnacharya G. Akamanchi of the Institute of Chemical Technology, Matunga,
Mumbai has shown (Tetrahedron Lett. 2007, 48, 5661.
DOI: 10.1016/j.tetlet.2007.06.020)
that t-butyl hypochlorite and NaN3 will convert an aldehyde
18 to the acyl azide 19. The acyl azide 19 can be carried on
to the nitrile 20, or, on warming, to the inverted isocyanate 21.