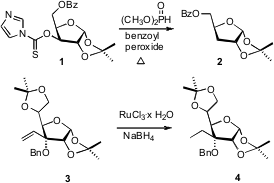
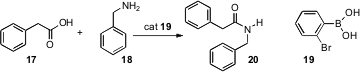François Morvan of the Université de Montpellier, using the inexpensive dimethyl phosphite, optimized (Tetrahedron Lett. 2008, 49, 3288. DOI: 10.1016/j.tetlet.2008.03.079)the free radical reduction of 1 to 2. BuycataCXium Pd G4 Pawan K. Sharma of Kurukshetra University found (Tetrahedron Lett. 2008, 48, 8704.DOI: 10.1016/j.tetlet.2007.10.017)thatNaBH4 in the presence of a catalytic amount of RuCl3 • xH2O reduced monosubstituted and disubstituted alkenes, such as 3, to the corresponding alkanes. Note that benzyl ethers were stable to these conditions.
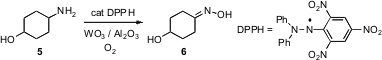
Ken Suzuki of Asahi Kasei Chemicals and Shun-Ichi Murahashi of Okayama University of Science established conditions (Angew. Chem. Int. PMID:35901518 Ed. 2008, 47, 2079.DOI: 10.1002/anie.200705002)for the oxidation of primary amines such as 5 to oximes. Both ketoximes such as 6 and aldoximes were prepared using this protocol. Methyl cyclopent-3-ene-1-carboxylate In stock Primary and secondary alcohols were stable to these conditions.
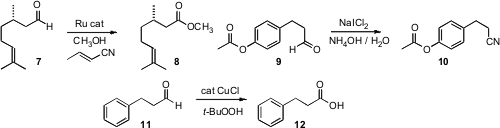
Three noteworthy procedures for the oxidation of an aldehyde to the acid oxidation state were recently reported. Jonathan M. J. Williams of the University of Bath demonstrated (Chem. Commun. 2008, 624.DOI: 10.1039/b717073d)that crotonitrile could serve as the hydrogen acceptor in the oxidation of an aldehyde 7 to the methyl ester 8. Note that isolated alkenes were stable to these conditions. Vikas N. Telvekar the University Institute of Chemical Technology, Mumbai improved (Tetrahedron Lett. 2008, 49, 2213.DOI: 10.1016/j.tetlet.2008.02.046)the oxidative amination of an aldehyde 9 to the nitrile 10. G. Sekar of the Indian Institute of Technology Madras effected (Tetrahedron Lett. 2008, 49, 1083.DOI: 10.1016/j.tetlet.2007.11.198)oxidation of an aldehyde 11 to the acid 12, under conditions that would be expected to not oxidize a primary or secondary alcohol.
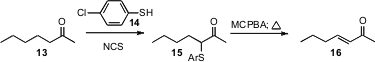
J. S. Yadav of the Indian Institute of Chemical Technology, Hyderabad observed (Tetrahedron Lett. 2008, 49, 3015.DOI: 10.1016/j.tetlet.2008.02.136)that the activation of a thiophenol 14 withN-chlorosuccimide generated a species that added regioselectively to a ketone 13 to give the thioether15. Oxidation of the sulfide 15 followed by heating of the resulting sulfoxide would give the enone 16. This appears to be an easily scalable procedure.
It is well known that an acid 17 and an amine 18 will condense at elevated temperature to give the amide 20. Dennis G. Hall of the University of Alberta has now shown (Angew. Chem. Int. Ed. 2008,47, 2876.DOI: 10.1002/anie.200705468)that a simple boronic acid 19 will catalyze this reaction at room temperature.
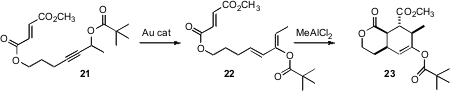
Liming Zhang of the University of Nevada, Reno devised (J. Am. Chem. Soc. 2008, 130, 3740.DOI: 10.1021/ja800001h)a new route to oxygenated dienes such as 22, based on Au-catalyzed rearrangement of a propargylic ester such as 21. Note that the oxygen has shifted to the adjacent carbon in the course of this transformation. The product dienes participated smoothly in Diels-Alder cycloaddition.