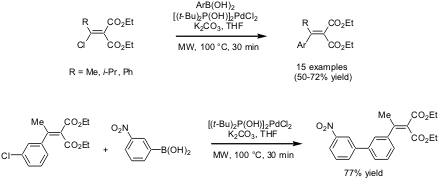
A recent publication by Nicholas J. PMID:26644518 Buy3-Azidopropylamine Turner and co-workers from the University of Edinburgh (J. Org. Chem. [(3-Bromocyclobutoxy)methyl]benzene Formula 2004, 69, 6920.DOI: 10.1021/jo049132r)describes the microwave-assisted palladium-catalyzed cross-coupling of β-chloroalkylidene/arylidene malonates ("vinyl chlorides") with boronic acids in a Suzuki-type fashion. The Suzuki arylation reaction was found to proceed with a wide range of arylboronic acids including electron-rich, electron-deficient and sterically demanding systems. For most reactions the presence of 1 mol% of the air-stable palladium catalyst [(t-Bu)2P(OH)]2PdCl2 provided a high yield of the desired β-aryl/alkylarylidene malonates. The catalyst was also found active to promote standard Suzuki reactions of aryl chlorides under microwave conditions.
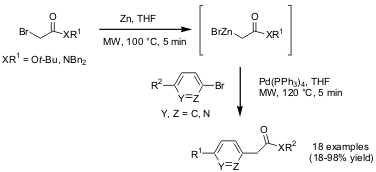
α-Arylation of Esters Using Reformatsky Reagents
Mark G. Moloney and co-workers from Oxford University (Tetrahedron Lett. 2004, 45, 7395.DOI: 10.1016/j.tetlet.2004.08.074)have reported the palladium-catalyzed α-arylation of esters and amides with organozinc reagents in a one-pot fashion. The requiredReformatsky reagents were readily prepared by microwave irradiation of the corresponding bromoacetate or bromoacetamide with zinc in THF for 5 min at 100 °C. Addition of this Reformatsky reagent to the coupling partner, an aryl bromide and the relevant catalyst/ligand in THF followed by further irradiation for 5-10 min at 120 °C provided the arylacetic esters or amides in good yields.
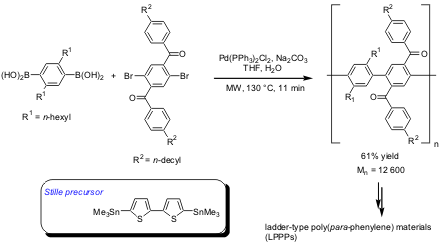
Ladder Polymers via Suzuki and Stille Couplings
The synthesis of fully conjugated semiconducting para-phenylene ladder polymers via microwave-assisted palladium-mediated "double" Suzuki and Stille-type reactions has been demonstrated by Ullrich Scherf and co-workers from Germany (Adv. Funct. Mater. 2004, 14, 352.DOI: 10.1002/adfm.200400010). Compared to the classical thermal protocols, reaction times were reduced by orders of magnitude and the molecular weight distributions of the polymers were changed significantly comparing microwave and thermal runs.
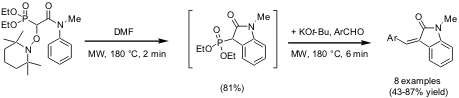
Homolytic Aromatic Substitution/Horner-Wadsworth-Emmons Olefinations
An efficient one-pot sequence comprising a homolytic aromatic substitution followed by an ionic Horner-Wadsworth-Emmons olefination for the production of a small library of α,β-unsaturated oxindoles was disclosed by Armido Studer and co-workers from The Philipps-Universität Marburg, Germany (Org. Lett. 2004, 6, 3477.DOI: 10.1021/ol048759t). SuitableTEMPO-derived alkoxyamine precursors were exposed to microwave irradiation DMF for 2 min to generate an oxindole intermediate via a radical reaction pathway (intramolecular homolytic aromatic substitution). After addition of KOt-Bu base and a suitable aromatic aldehyde the mixture was exposed again to microwave irradiation at 180 °C for 6 min to provide the α,β-unsaturated oxindoles in moderate to high overall yields.