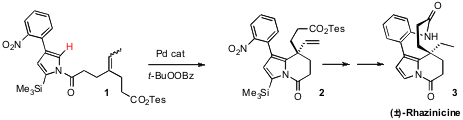
The power of catalytic C-H functionalization is illustrated by the elegant synthesis of rhazinicine (3) devised (Angew. Chem. PMID:34235739 Int. Ed. 2008, 47, 3004. DOI: 10.1002/anie.200705005)by Matthew J. Propargyl-PEG1-NHS ester Chemical name Gaunt of the University of Cambridge. The key step in the synthesis was the oxidative cyclization of 1 to 2. Although 1 has many C-H sites, the Pd catalyst selected for the α position of the pyrrole, leading, after intramolecularHeck addition and β-hydride elimination, to the alkene 2. Reduction and macrolactamization completed the synthesis of 3.
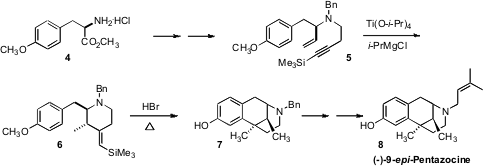
Hongbin Zhai of the Shanghai Institute of Organic Chemistry and Zhong Li of East China University of Science and Technology prepared (Org. Lett. 2-(3-Fluoro-2-methoxyphenyl)acetic acid Chemscene 2008, 10, 2457. DOI: 10.1021/ol800737d)the analgesic (-)-9-epi-pentazocine 8 from the amino ester4, itself available from D-tyrosine. In the conversion of 5 to 6, the (i-PrO)2Ti formed a ring, leading to 6 as a single diastereomer and geometric isomer. HBr then effected both deprotection of the methyl ether and cyclization, to give 7, which was carried on to 8.
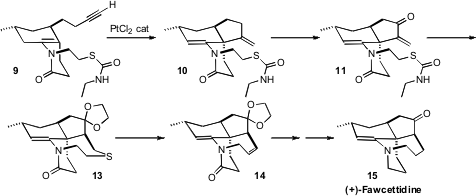
The Pt-catalyzed cyclization of 9 to 10 set the stage for the synthesis of (+)-fawcettidine (15) by Gregory R. Dake of the University of British Columbia (Angew. Chem. Int. Ed. 2008, 47, 4221.DOI: 10.1002/anie.200800522). This synthesis also illustrated the power of the Ramberg-Bäcklund reaction for the assembly of medium rings. The thiolate liberated from 12 readily added to the enone, to give 13. Oxidation to the sulfone followed by the Ramberg-Bäcklund reaction (halogenation, intramolecular displacement, chelotropic elimination of SO2) then delivered 14, which was selectively reduced, leading to 15.
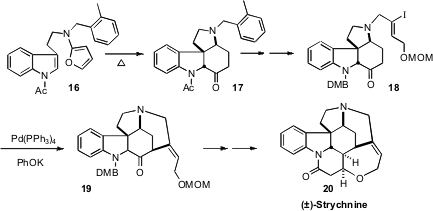
Albert Padwa of Emory University has developed (J. Org. Chem. 2008, 73, 3539. DOI: 10.1021/jo8003716)a general route to the Strychnos alkaloids, based on the facile cyclization of the furan 16 to the tetracyclic ketone 17. This project culminated in the synthesis of the heptacyclic strychnine 20.
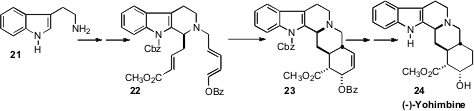
Eric N. Jacobsen of Harvard University has devised a family of catalysts for the enantioselective Pictet-Spengler reaction of tryptamine 21. He has now (Org. Lett. 2008, 10, 745.DOI: 10.1021/ol702781q)used this approach to prepare the triene22 in 94% ee. The Diels-Alder cyclization of 22 proceeded with high diastereocontrol to give23, the immediate precursor of (-)-yohimbine 24.