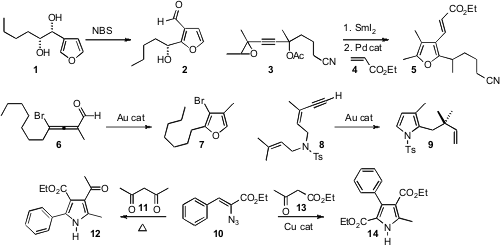
Several new routes to furans and to pyrroles have recently been put forward. Inspired by the Achmatowicz ring expansion, Patrick J. Walsh of the University of Pennsylvania developed (J. Am. Chem. Soc. 2008, 130, 4097. DOI: 10.1021/ja710988q)the oxidative rearrangement of 3-hydroxalkyl furans such as 1 to the 3-aldehyde 2. 6-Fluoroindolizine-2-carboxylic acid Order José M. Aurrecoechea of the Universidad del País Vasco established (J. Org. Chem. 2008, 73, 3650.DOI: 10.1021/jo800211q)that cumulated alcohols, available by reduction of alkynes such as 3 withSmI2, rearrange under Pd catalysis, and then add to an acceptor alkene such as 4, to give the furan 5. PMID:34856019 Vladimir Gevorgyan of the University of Illinois at Chicago used (J. Am. Chem. Soc. 1-(2,2,2-Trifluoroethyl)piperazine site 2008, 130, 1440.DOI: 10.1021/ja0773507)an Au catalyst to rearrange an allene such as 6 to the bromo furan 7. Fabien L. Gagosz of the Ecole Polytechnique, Palaiseau, also found (Org. Lett. 2007, 9, 3181.DOI: 10.1021/ol0713032)that an Au catalyst rearranged the eneyne 8 to the pyrrole 9.
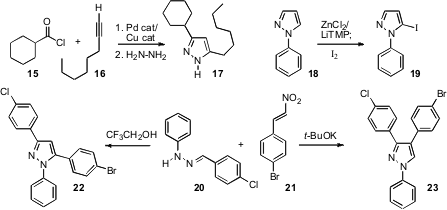
Azido esters such as 10 are readily prepared from the corresponding aldehyde by phosphonate condensation. Shunsuke Chiba and Koichi Narasaka of Nanyang Technology University demonstrated (Org. Lett. 2008, 10, 313. DOI: 10.1021/ol702727j)that thermal condensation of 10 with acetyl acetone 11 gave the pyrrole12, while Cu catalyzed condensation with acetoacetate 13 gave the complementary pyrrole 14.
Huan-Feng Jiang of South China University of Technology observed (Tetrahedron Lett. 2008, 49, 3805.DOI: 10.1016/j.tetlet.2008.03.153)that condensation of an acid chloride 15 with an alkyne 16, presumably to give the alkynyl ketone, followed by the addition of hydrazine delivered the pyrazole 17. Masanobu Uchiyama of RIKEN and Florence Mongin of the Université de Rennes 1 established (J. Org. Chem. 2008,73, 177. DOI: 10.1021/jo7020345)that a pre-formed pyrazole 18 could be metalated and then iodinated, to give 19. Xiaohu Deng of Johnson & Johnson, San Diego reported (Org. Lett. 2008, 10, 1307,DOI: 10.1021/ol800200j;J. Org. Chem. 2008, 73, 2412,DOI: 10.1021/jo7026195)complementary routes to pyrazoles, combining 20 and 21 under acidic conditions to give 22, and under basic conditions to give 23.
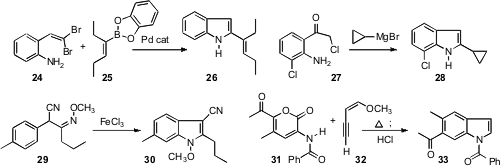
Mark Lautens of the University of Toronto demonstrated (J. Org. Chem. 2008, 73, 538.DOI: 10.1021/jo701987r)that dibromoalkenes such as 24, readily available from the corresponding aldehyde, could be condensed with an organoborane such as 25 to give the indole 26. Tao Pei and Cheng-yi Chen of Merck Process established (Angew. Chem. Int. Ed. 2008, 47, 4231.DOI: 10.1002/anie.200705804)that the addition of an organometallic to the ketone 27 drove rearrangement to a homologated ketone, that on acid work-up gave the indole 28.
Each of these approaches depended on the availability of the ortho-substituted aniline starting materials. Junbiao Chang and Kang Zhao of Tianjin University devised (J. Org. Chem. 2008, 73, 2007. DOI: 10.1021/jo7024477)a complementary approach, the cyclization of 29 to 30, in the process directly aminating the benzene ring. Marijan Kocevar of the University of Ljubljana established (Tetrahedron 2008, 64, 45.DOI: 10.1016/j.tet.2007.10.099)an alternative Diels-Alder approach to indoles, combining 31 and 32 to give 33.
The preparation of pyridines will be covered in the next column on heteroaromatic construction.