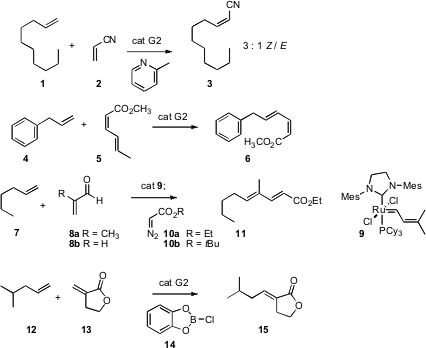
Alkene metathesis has been extended to increasingly complex starting materials and products. Nitriles are good donors to coordinatively-unsaturated transition metal centers, so tend to inhibit the reaction. PMID:35345980 Ren He of Dalian University of Technology has found (Tetrahedron Lett. 2007, 48, 4203. DOI: 10.1016/j.tetlet.2007.04.065)that inclusion of the loosely-coordinating 2-methyl pyridine in the reaction enables facile cross-coupling with acrylonitrile (2). Ribavirin manufacturer Althoughcross-coupling with (Z,Z)-sorbate is not efficient, Dennis P. Curran of the University of Pittsburgh has shown (Org. 947725-04-4 custom synthesis Lett. 2007, 9, 5. DOI: 10.1021/ol062017d)that cross-coupling with (E,Z)-sorbate (5) works well. For large scale work, he has developed a Hoveyda-type catalyst with a perfluoro tail, that is recoverable in 70% recrystallized yield from the reaction mixture. Shigefumi Kuwahara of Tohoku University has reported (Tetrahedron Lett. 2007, 48, 3163. DOI: 10.1016/j.tetlet.2007.03.055)a practical alternative for direct metathesis to deliver (E,E)-dienyl esters.
Continuing the investigation of tandem Ru-catalyzed reactions, Marc L. Snapper of Boston College effected (Org. Lett. 2007, 9, 1749. DOI: 10.1021/ol070445t)metathesis with methacrolein (8a), then added Ph3P and diazoacetate, to give the diene 11. A range of common Ru catalysts worked well for this transformation. In an alternative approach to trisubstituted alkene construction, Stellios Arseniyadis and Janine Cossy of ESPCI Paris have demonstrated (Org. Lett. 2007, 9, 1695. DOI: 10.1021/ol0703940)that inclusion of Cl-catecholborane (14) allows clean cross metathesis with the lactone 13.
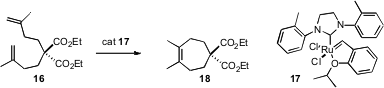
The construction of tetrasubstituted alkenes has been more challenging. Yann Schrodi of Materia, Inc. (Org. Lett. 2007, 9, 1589. DOI: 10.1021/ol0705144)has described a catalyst 17 that is particularly effective. Complex 17 was superior to a catalyst reported (Org. Lett. 2007, 9, 1339. DOI: 10.1021/ol070194o)shortly earlier by Robert H. Grubbs of Caltech.
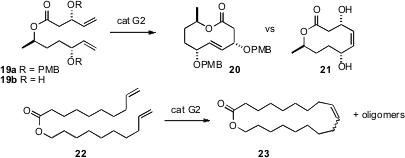
Debendra K. Mohapatra of the National Chemical Laboratory, Pune, and Professor Grubbs, in a new approach to macrocyclic stereocontrol, have made (Tetrahedron Lett. 2007, 48, 2621. DOI: 10.1016/j.tetlet.2007.02.040)the remarkable observation that the cyclization of the bis ether 19a gave 20 in a 9:1 E / Z ratio, while cyclization of the diol 9b gave only Z–21. Oligomer formation can often compete in such medium ring-forming reactions. Deryn E. Fogg of the University of Ottawa has raised (J. Am. Chem. Soc. 2007, 129, 1024. DOI: 10.1021/ja067531t)the cautionary (but happy!) observation that while the cyclization, for instance, of 22 proceeded efficiently to give23, at an intermediate point in the transformation the product was more than half oligomer.
Removal of Ru residue at the end of the reaction is always an issue. Professor Grubbs has decribed (Org. Lett. 2007, 9, 1955. DOI: 10.1021/ol070512j)a PEG-based catalyst that is easy to separate from the metathesis products. Alternatively, Steven T. Diver of the University at Buffalo has shown (Org. Lett. 2007, 9, 1203. DOI: 10.1021/ol0631399)that workup of the reaction mixture from G1 or G2 with a polar isocyanide led to products having less than 5 ppm Ru.