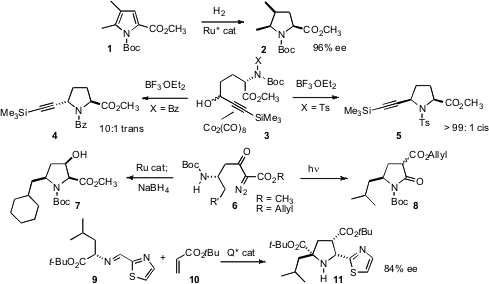
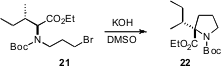Ryoichi Kuwano of Kyushu University showed (J. Am. 1-Hydroxyhept-6-yn-3-one structure Chem. Formula of 4-Bromo-3-fluoropicolinaldehyde Soc. 2008,130, 808. DOI: 10.1021/ja7102422)that diastereomerically and enantiomerically pure pyrrollidines such as 2 could be prepared by hydrogenation of the corresponding pyrrole. Victor S. Martín of Universidad de la Laguna found (Org. PMID:23376608 Lett. 2008, 10, 2349. DOI: 10.1021/ol800544a)that the stereochemical outcome of the pyrrolidine-forming Nicholas cyclization could be directed by the protecting group on the N. Jianbo Wang of Peking University established (J. Org. Chem. 2008, 73, 1971. DOI: 10.1021/jo702275a)a convenient route to diazo esters such as 6. N-H insertion led to the pyrrolidine, which Zhen-Jiang Xu of the Shanghai Institute of Organic Chemistry and Chi-Ming Che of the University of Hong Kong showed (Org. Lett. 2008, 10, 1529. DOI: 10.1021/ol800087p)could be reduced with high diastereoselectivity to the hydroxy ester 7. Alternatively, Professor Wang found that photochemicalWolff rearrangement of 6 delivered the pyrrolidone 8. Martin J. Slater and Shiping Xie of GlaxoSmithKline optimized (J. Org. Chem. 2008, 73, 3094. DOI: 10.1021/jo800062c)the hydroquinine catalyzed enantioselective3+2 cycloaddition of 9 and 10, leading to the pyrrolidine 11 with high diastereocontrol.
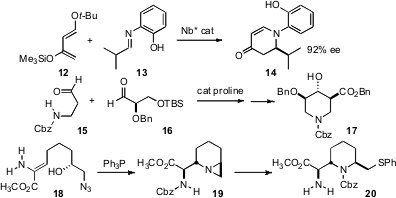
Shu Kobayashi of the University of Tokyo developed (Adv. Synth. Catal. 2008, 350, 647. DOI: 10.1002/adsc.200700615)a practical protocol for the azaDiels-Alder construction of enantiomerically-purepiperidines such as 14. Biao Yu of the Shanghai Institute of Organic Chemistry cyclized (Tetrahedron Lett. 2008, 49, 672. DOI: 10.1016/j.tetlet.2007.11.153)the product from the proline-catalyzed enantioselective aldol of 15 and 16, leading to the substituted piperidine 17. Michael Shipman of the University of Warwick described (Tetrahedron Lett. 2008, 49, 250. DOI: 10.1016/j.tetlet.2007.11.077)the cyclization of the aziridine derived from 18, that proceeded to give 19 as a single diastereomer, apparently via kinetic side-chain protonation.
Takeo Kawabata of Kyoto University found (J. Am. Chem. Soc. 2008,130, 4153. DOI: 10.1021/ja077684w)that intramolecular alkylation to form four, five and six-membered rings from amino esters such as 21 proceeded with remarkable enantioretention.
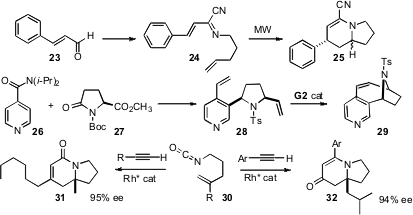
Géraldine Masson and Jieping Zhu of CNRS, Gif-sur-Yvette, condensed (Org. Lett. 2008, 10, 1509. DOI: 10.1021/ol800199b)cinnamaldehyde (23) with cyanide and an ω-alkenyl amine to give the intramolecular aza-Diels-Alder substrate 24. Hongbin Zhai of the Shanghai Institute of Organic Chemistry acylated (J. Org. Chem. 2008, 73, 3589.DOI: 10.1021/jo8002425)26 with27, leading to the ring-closing metathesis precursor 28. Tomislav Rovis of Colorado State University developed (Org. Lett. 2008, 10, 1231.DOI: 10.1021/ol800086s)the Rh-catalyzed condensation of the isocyanate30 with alkyl alkynes to give 31, and with aryl alkynes to give 32.