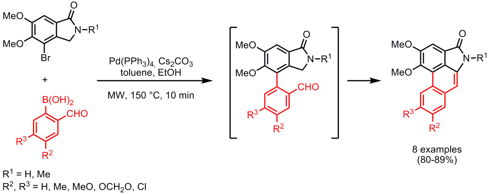
The group of Christian Wolf from Georgetown University, Washington, has reported on the synthesis of benzophenone and acetophenone derivatives via the Suzuki-type cross-coupling of aromatic and aliphatic acyl chlorides with boronic acids that thus overcomes the drawbacks of Friedel-Crafts acylations such as harsh conditions, untunable regiocontrol and narrow substrate scope (Tetrahedron Lett. 2008, 49, 5773.DOI: 10.1016/j.tetlet.2008.07.115). 7-Bromo-1H-indole-6-carbonitrile Formula When using palladium-phosphinous acid, POPd, as catalyst system in combination with microwave heating, the ketone products are obtained in good to high yields in 10 min reaction time (vs. 1 h at the same temperature under thermal heating). Importantly, aryl halides in both the acyl chloride and boronic acid substrate were tolerated. PMID:23795974 1346245-52-0 Formula
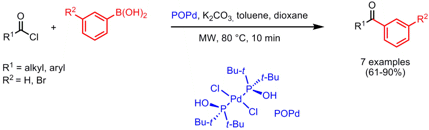
Synthesis of Tertiary Amides
Simon Woodward and co-workers from the University of Nottingham have demonstrated the direct coupling of cyclic secondary amines with esters employing the air-stable trimethylaluminium source DABAL-Me3 for the preparation of tertiary amides (Tetrahedron Lett. 2008, 49, 5687. DOI: 10.1016/j.tetlet.2008.07.090). Microwave heating at 130 °C for 5-16 min afforded the amides in good to excellent yields, with the longer reaction times necessary for more hindered amine-ester pairs. Weinreb amides can be also prepared in good yields by using a one-pot in situ deprotonation with NaH to liberate the free N-methoxy-N-methylamine followed by the coupling step.
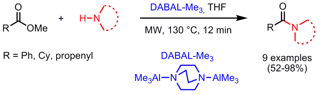
Synthesis of Indole-Fused 1,4-Diazepines
The group of Nobutaka Fujii and Hiroaki Ohno from Kyoto University has developed a Cu(I)-catalyzed domino three-component coupling/indole formation/N-arylation protocol for the synthesis of indole-fused 1,4-diazepines (Org. Lett. 2008, 10, 3535.DOI: 10.1021/ol801383b). The first step toward the indole-fused tetracylic scaffolds is a Mannich type reaction of 2-ethynylanilines, o-bromobenzylamines and paraformaldehyde, followed by the indole formation, subsequent deprotection of the N-mesyl group by addition of MeONa and the final N-arylation. In addition to the fused benzodiazepines, heterocycle-fused 1,4-diazepines with a pyridine orthiophene moiety were also prepared.
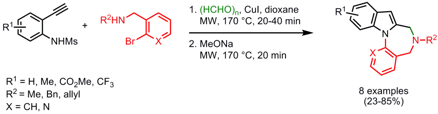
Total Synthesis of Aristolactams
Jung-Nyoung Heo and his group from the Korea Research Institute of Chemical Technology have published a Suzuki-Miayura coupling/aldol condensation cascade reaction as the key step in the total synthesis of natural and unnatural aristolactams (Org. Lett. 2008, 10, 3543.DOI: 10.1021/ol801291k). In this one-pot synthesis 4-bromoisoindolin-1-one is reacted with various 2-formylphenylboronic acids using Pd(PPh3)4 as catalyst, Cs2CO3 as base and toluene/EtOH as solvent system to construct the core phenanthrene ring system. Only when 3-thienylboronic acid is employed, a lower product yield of 35% was obtained.
.