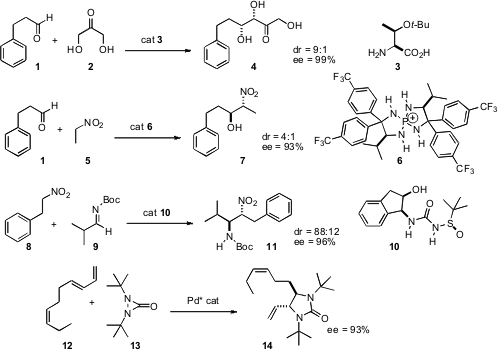
An impressive array of new catalysts for enantioselective homologation have been reported. Carlos F. Barbas III of Scripps/La Jolla has found (Angew. Chem. Int. Ed. 2007, 46, 5572. DOI: 10.1002/anie.200701269)that the commercial amino acid 3 mediated the addition of dihydroxyacetone 2 to an aldehyde such as 1 to give the triol 4 with high enantio- and diastereocontrol. Takashi Ooi of Nagoya University has devised (J. Am. Chem. Soc. 2007, 129, 12392. DOI: 10.1021/ja075152+)the catalyst 6 for the anti addition (Henry reaction) of nitro alkanes such as 5 to aldehydes. Takayoshi Arai of Chiba University has developed (Org. Lett. 2007,9, 3595. DOI: 10.1021/ol7014362)a complementary catalyst (not shown) that mediated syn addition. Jonathan A. Ellman of the University of California, Berkeley has uncovered (J. Am. Chem. Soc. 2007, 129, 15110. 3-Bromo-5-methoxyphenol manufacturer DOI: 10.1021/ja075653v)the catalyst 10 for the aza-Henry reaction. Yian Shi of Colorado State University has found (J. PMID:23800738 Am. Chem. 287193-01-5 web Soc. 2007, 129, 11688. DOI: 10.1021/ja074698t)ligands for Pd that direct the absolute sense of the addition of 13 to dienes such as 12.
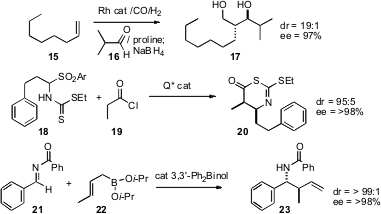
Bernhard Breit of Albert-Ludwigs-Universität, Freiburg has devised conditions (Adv. Synth. Catal. 2007, 349, 1891. DOI: 10.1002/adsc.200700216)for the Rh-catalyzed hydroformylation of α-olefins such as 15, and same-pot proline-catalyzed condensation of the linear aldehyde so produced with a branched aldehyde such as 16 to give, after reductive workup, the branched diol17. Scott G. Nelson of the University of Pittsburgh has established (J. Am. Chem. Soc. 2007, 129, 11690.DOI: 10.1021/ja074845n)conditions, using Cinchona alkaloid derived catalysts, for the condensation of the imine surrogate 18 with the ketene precursor 19, to give the Mannich product 20. Scott E. Schaus of Boston University has developed (J. Am. Chem. Soc. 2007, 129, 15398.DOI: 10.1021/ja075204v)a complementary approach, based on catalyzed addition of isolatedallyl boronates such as 22 to the activated imine21. Kálmán J. Szabó of Stockholm University has found (J. Am. Chem. Soc. 2007, 129, 13723.DOI: 10.1021/ja074917a)that substituted allyl boronates can be prepared and reacted in situ.
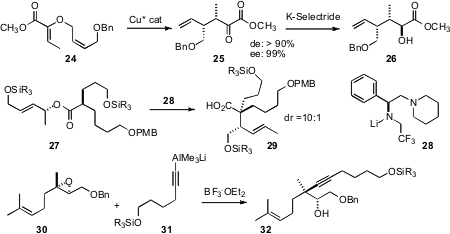
Martin Hiersemann of the Universität Dortmund has reported (Org. Lett. 2007, 9, 4979.DOI: 10.1021/ol702092h)the remarkable Cu*-catalyzedClaisen rearrangement of the prochiral 24, leading to 25 and thus to the versatile intermediate26. At least as remarkable is the Claisen rearrangement, mediated by the matched enantiomerically-pure base 28, of 27 to 29, developed (Angew. Chem. Int. Ed. 2007, 46, 7466.DOI: 10.1002/anie.200702142)by Armen Zakarian, now at the University of California, Santa Barbara. Brian L. Pagenkopf of the University of Western Ontario has devised (Tetrahedron2007, 63, 8774.DOI: 10.1016/j.tet.2007.06.036)a general route to both cyclic and acyclic alkylated stereogenic centers, by opening trisubstituted epoxides such as 30 with alkynes.