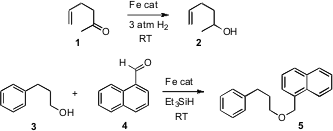
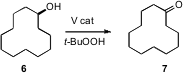Although methods both for reduction and for oxidation are well developed, there is always room for improvement. PMID:24059181 While ketones are usually reduced using metal hydrides, hydrogen gas is much less expensive on scale. 1346245-52-0 Purity Charles P. Casey of the University of Wisconsin has devised (J. Am. Chem. Soc. 2007,129, 5816. DOI: 10.1021/ja071159f)an Fe-based catalyst that effects the transformation of 1 to 2. Note that the usually very reactive monosubstituted alkene is not reduced and does not migrate. Takeshi Oriyama of Ibaraki University has developed a catalyst, also Fe-based (Chemistry Lett. 2007, 38.DOI: 10.1246/cl.2007.38)for reducing aldehydes to ethers. Using this approach, an alcohol such as 3 can be converted into a variety of substituted benzyl ethers, including 5. Simple aliphatic aldehydes and alcohols also work well.
Oxidation of alcohols to aldehydes or ketones is one of the most common of organic transformations. 167073-08-7 Formula Several new processes catalytic in metal have been put forward. Tharmalingam Punniyamurthy of the Indian Institute of Technology, Guwahati has found (Adv. Synth. Catal. 2007, 349, 846.DOI: 10.1002/adsc.200600397)that catalytic V(IV) oxide on silica gel, stirred with t-butyl hydroperoxide in t-butyl alcohol at room temperature smoothly oxidized 6 to 7. After the reaction, the catalyst was separated by filtration. Another carbonyl can also serve as the hydride acceptor, but then the transfer can be reversible.
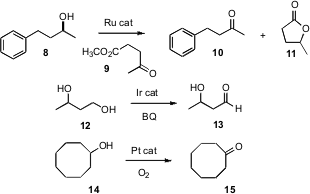
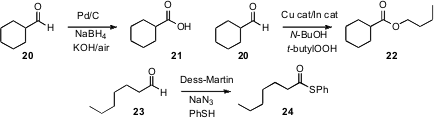
Jonathan M. J. Williams of the University of Bath has shown (Tetrahedron Lett. 2007, 48, 3639.DOI: 10.1016/j.tetlet.2007.03.135)that with a Ru catalyst, methyl levulinate (9) could serve as the hydride acceptor, with the byproduct alcohol being drained off as the lactone 11. Hansjörg Grützmacher of the ETH Zürich developed an Ir catalyst (Angew. Chem. Int. Ed. 2007, 46, 3567.DOI: 10.1002/anie.200605170), that showed marked preference for the oxidation of primary over secondary alcohols with benzoquinone as the net oxidant. Yasuhiro Uozumi of the Institute for Molecular Science, Aichi, has devised (Angew. Chem. Int. Ed. 2007,46, 704.DOI: 10.1002/anie.200603900)a nanoencapsulated Pt catalyst that worked well withO2 or even with air. The catalyst was easily separated from the product, and maintained its activity over several cycles.
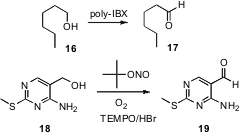
Oxidation can also be carried out without metal catalysis, but this requires stoichiometric reagent, so excess reagent and byproducts must be separated. Yoon-Sik Lee of Seoul National University has described (Tetrahedron Lett. 2007, 48, 3731.DOI: 10.1016/j.tetlet.2007.03.089)the preparation of an easily-swellable polymerichypervalent iodine reagent that oxidized alcohols to aldehydes and ketones, including 16 to 17. The spent reagent was removed by filtration. An alternative is to use volatile/water soluble reagents. Xinquan Hu of the Zhejiang University of Technology has devised (J. Org. Chem. 2007, 72, 4288.DOI: 10.1021/jo0705824)a combination of HBr, oxygen and t-butyl nitrite that, with catalyticTEMPO, oxidized 18 to 19. This inexpensive protocol was easily scaled.
Three procedures for the oxidation of aldehydes to acids have recently appeared. Gwangil An of the Korea Institute of Radiological and Medical Sciences and Hakjune Rhee of Hanyang University have described (Tetrahedron Lett. 2007, 48, 3835.DOI: 10.1016/j.tetlet.2007.03.151)conditions leading to the carboxylic acid, Chao-Jun Li of McGill University has developed (Tetrahedron Lett. 2007, 48, 1033.DOI: 10.1016/j.tetlet.2006.11.169)an aldehyde to ester conversion that accommodated methanol as well as a range of primary and secondary alcohols, and B. P. Bandgar of Swami Ramanand Teerth Marathwada University has found (Tetrahedron Lett. 2007, 48, 1287. DOI: 10.1016/j.tetlet.2006.12.024)conditions for the oxidation of an aldehyde directly to the thioester.