The group of Stefan Bräse from the University of Karlsruhe has performed optimization studies toward solvent, time, temperature and catalyst loading for the microwave-mediated organocatalytic asymmetric α-amination of disubstituted aldehydes with diethyl azodicarboxylate (Eur. J. Org. Chem. 922718-57-8 site 2008, ASAP. 1,4-Dihydropyrazine-2,3-dithione Price DOI: 10.1002/ejoc.200701167). PMID:26895888 Optimum conditions in terms of both high yield and enantioselectivity proved to be MeCN as solvent, 50 mol% of L-proline as catalyst, a reaction temperature of 60°C and 30 min reaction time. Importantly, the reaction time could be decreased from several days to only 30 min by applying microwave heating compared to room temperature conditions, with a concomitant increase in yield and enantioselectivity. In particular, improvements for otherwise unreactive starting materials bearing electron-withdrawing R-groups (e.g. F, NO2, CF3) were observed.
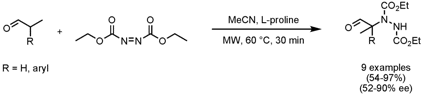
Nickel-on-Graphite Catalyzed Cross-Couplings
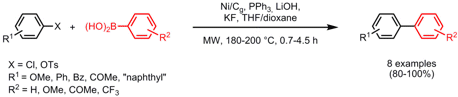
Carbon-carbon cross-couplings catalyzed by activated Ni(II) mounted on graphite (Ni/Cg) were reported by the group of Bruce Lipshutz from the University of California, Santa Barbara (Org. Lett. 2008, 10, 697. DOI: 10.1021/ol702453q). In addition to the Suzuki coupling of aryl chlorides and tosylates with boronic acids, vinylalanes and vinylzirconocenes were reacted with aryl halides and tosylates. Compared to the corresponding nickel-in charcoal (Ni/C) catalyzed reactions, couplings proceed faster, higher yields are obtained and cleaner reactions are afforded with Ni/Cg as catalyst. In some cases, different chemoselectivities depending on the used catalyst were obtained.
Desulfitative Sonogashira-Type Cross-Coupling
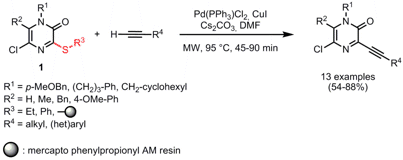
The desulfitative Sonogashira-type cross-coupling of thioether-functionalized pyrazinones 1 and various terminal alkynes was disclosed by Erik Van der Eycken and his group from the University of Leuven (Org. Lett. 2008,10, 1147. DOI: 10.1021/ol800054b). The reaction proceeded well for both ethylthio-substituted and resin-linked pyrazinones, although longer reaction times (60 and 90 min) compared to the phenylthio-substituted scaffolds are necessary for resin bound reactions. In addition, the desulfitative alkynylation was extended to other (het)aryls such as oxazinones, pyrazines and phenyl thioesters.
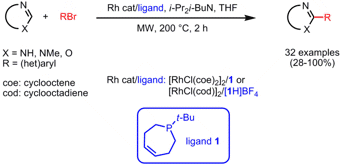
Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation
In order to overcome problems such as substrate scope and functional group tolerance in metal-catalyzed direct arylations and to improve practicability of previously published Rh-catalyzed arylations, the groups of Robert Bergman and Jonathan Ellman from the University of California, Berkeley, have developed a new protocol for the direct arylation of a variety of heterocycles with aryl bromides employing phosphepine ligand1 (J. Am. Chem. 2008, 130, 2493. DOI: 10.1021/ja0748985). This ligand proved to be the most effective in a ligand screen and coordinates to Rh in a bidentate P-olefin fashion and thus generates an active and temperature stable catalyst. For a greater practicability of the protocol, the air-sensitive ligand 1 can be protected as its HBF4 salt and [RhCl(cod)]2 as air-stable Rh-source can be employed. Thus, reaction mixture preparation in a glovebox is avoided and only an inert atmosphere in the microwave vial is required. In addition, by using THF as low boiling solvent product isolation is simplified.
.