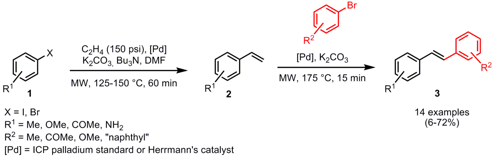
John Verkade and Steven Raders from Iowa State University have reported on the synthesis of diaryl ethers by the reaction of electron deficient aryl fluorides with variousTBDMS-protected phenols (Tetrahedron Lett. 2008,49, 3507.DOI: 10.1016/j.tetlet.2008.03.089). Proazaphosphatrane 1 was utilized as strong base in this protocol, and compared to conventional heating the amount could be reduced from 10-50 mol% to only 1-10 mol% giving the products in comparable high yields. For NO2-substituted aryl fluorides toluene was used as solvent whereas the other aryl fluorides required DMF. PMID:23554582
Hydrogenation of Pyridines
The group of Maurizio Taddei from the Università degli Studi di Siena, Italy, has investigated the preparation of substitutedpiperidines via hydrogenation of the corresponding pyridine derivatives (Synlett 2008, 1125-1128.DOI: 10.1055/s-2008-1072717). 728034-12-6 In stock For introducing hydrogen into the microwave vial a special gas charging accessory for the dedicated microwave instrument was employed. Price of 1417789-17-3 Key to the success of reproducible hydrogenations was the pre-reduction of PtO2 to Pt in acetic acid with hydrogen combined with microwave heating at 50 °C for 15 min. Subsequent addition of the pyridine building block, charging the vial again with hydrogen and heating at 80 °C delivered the piperidine products. Importantly, the hydrogenation was as stereoselective as hydrogenations carried out at room temperature.

Copper-Catalyzed One-Pot Benzoxazole Synthesis
A 24-member benzoxazole library was synthesized by Robert Batey and coworkers from the University of Toronto using an automated sequential processing technique (J. Org. Chem. 2008, 73, 3452.DOI: 10.1021/jo702145d). The reaction proceeds in one pot via initial acylation of the aniline building blocks with acid chlorides giving the 2-haloanilide intermediates 1, followed by copper-catalyzed intramolecular cyclization of 1 to form the C-O bond of the benzoxazole products. Shorter reaction times could be achieved by employing microwave heating compared to conventional heating (15 min at 210 °C vs. 24 h at 95 °C).
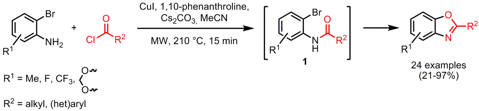
Synthesis of Nonsymmetrically Substituted Stilbenes
Nicholas Leadbeater and Chad Kormos from the University of Connecticut have disclosed a one-pot two-step Heck coupling strategy for the preparation of nonsymmetrically substituted stilbenes (J. Org. Chem. 2008, 73, 3854-3858. DOI: 10.1021/jo800235c). In the first step, styrenes 2 were synthesized selectively by employing ethene as coupling partner using a gas-loading device. For aryl iodides (1) as coupling partners, 0.02 mol% Pd using an ICP standard and 125 °C were required for obtaining the coupled products 2 whereas for aryl bromides (1) 0.5 mol% of the Herrmann´s catalyst and 150 °C are necessary. In the next step, styrenes 2 are coupled with aryl bromides using a 1:1 molar ratio and Herrmann´s catalyst in the same reaction vial to get stilbenes 3. It has to be noted that the less reactive aryl halide has to be used in the first coupling step in order to reduce the competitive symmetric stilbene formation and thus increase the product yield.
.