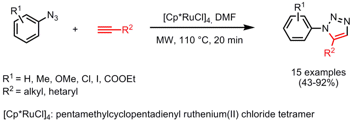
The synthesis of 1,2,3-triazoles via Ru-catalyzed aryl azide-alkyne cycloadditions was described by the group of Valery Fokin from The Scripps Research Institute (Org. Lett. 2007, 9, 5337. DOI: 10.1021/ol701912s). PMID:35991869 In comparison to the Cu(I)-catalyzedazide-alkyne cycloaddition where 1,4-disubstituted triazoles are obtained, the Ru-catalyzed version produces 1,5-regioisomers of 1,2,3-triazoles. (2-Cyanopyridin-3-yl)boronic acid Price [Cp*RuCl]4 proved to be superior in activity than the original Cp*RuCl(PPh3)2 catalyst and in combination with microwave heating higher yields, cleaner products and shorter reaction times were accomplished. 151763-88-1 Chemical name By-product formation and lower yields were noticed under conventional heating conditions in an oil bath.
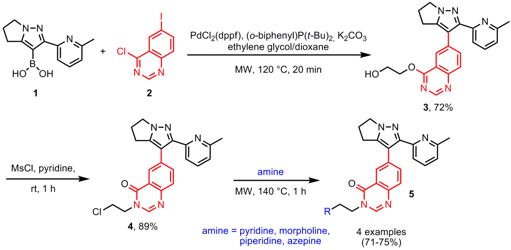
One-Pot Suzuki-Miyaura/Etherification Reaction
In the course of the synthesis of quinazolinone TGF-β RI inhibitors 5, Hong-yu Li and co-workers from the Lilly Corporate Center in Indianapolis have disclosed the synthesis of key intermediate 3 via a one-pot three-component Suzuki-Miyaura/etherification sequence (Tetrahedron 2007, 63, 11763. DOI: 10.1016/j.tet.2007.08.069). In order to prevent side reactions in the Suzuki coupling of boronic acid 1 and quinazoline2 (in particular Suzuki reaction at the 2-chloro position of 2), ethylene glycol was employed for the etherification step at the 2-position of 2. In addition, product purification was improved by performing the reaction sequence in one pot. The final products 5 were obtained by amination of scaffold4, again using microwave heating.
Synthesis of Potential β-Sheet Nucleators via Suzuki Coupling
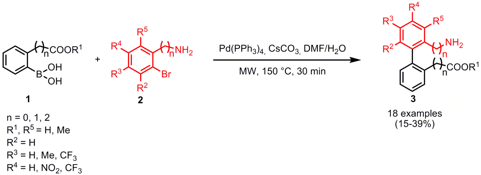
Vincenzo Santagada and his group from Universitá di Napoli “Federico II”, Italy, have reported on the synthesis of biphenyls 3 that incorporate an amino acid moiety with the carboxyl and amino function in ortho position (Tetrahedron2007, 63, 12779. DOI: 10.1016/j.tet.2007.09.071). These biphenyl scaffolds were synthesized in order to develop new compounds that are able to induce β-hairpin folding in peptides. Biphenyls 3 were obtained in a single step by the Suzuki coupling of boronic acids 1 and aniline (n = 0), benzylamine (n = 1) or phenethyl amine (n = 2) bromides 2 in aqueous DMF as solvent and 30 min microwave heating at 150 °C.
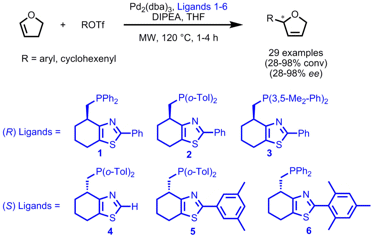
Asymmetric Heck Reactions using Phosphine-Thiazole Ligands
Pher Andersson and co-workers from the Department of Biochemistry and Organic Chemistry in Uppsala have made investigations toward the impact of the ligand structure on the asymmetric Heck coupling of 2,3-dihydrofuran with various triflates (Adv. Synth. Catal. 2007, 2595.DOI: 10.1002/adsc.200700390). With all employed phosphine-thiazole ligands 1-6 the regioselectivity was excellent whereas the enantioselectivity and the reaction rate were dependent on the substitution patterns of the ligands. For the coupling with aryl triflates bulkier substituents in the 2-position of the ligands enhanced the enantioselectivity and reaction rate: ligand 6 proved to be the best regardingee´s (94-98%) and reaction time (1 h) whereas with ligand 4 low conversions (28-30%) and ee´s (77-80%) were obtained. By employing microwave heating the reactions proceed faster with constant high enantioselectivities.
.