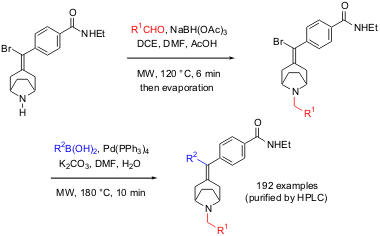
In the chemistry described below, Coats and a group of researchers from Johnson and Johnson utilized successive reductive aminations and Suzuki cross coupling reactions to prepare a 192-member library of tropanylidene benzamides (Bioorg. Med. Chem. Lett. 2004, 14, 5493.DOI: 10.1016/j.bmcl.2004.09.004). This series of tropanylidene opioid agonists proved extremely tolerant of structural variation while maintaining excellent opioid activity. For the solution phase preparation of functionalized tropanylidenes the authors simply dispensed 1,2-dichloroethane (DCE) solutions of the bromo N-H precursor to a set of microwave vials, added the aldehydes (3 equiv) and a solution of sodium triacetoxy borohydride in dimethylformamide (2 equiv), and subjected the mixture to microwave irradiation for 6 min at 120 °C. Quenching the reductive amination with water and subsequent concentration allowed to directly perform a microwave-assisted Suzuki reaction on the crude products. 2-Fluoro-3,4-dimethylbenzoic acid structure
Carbasugar Synthesis
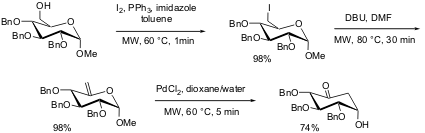
A rapid synthesis of carbasugars was reported by Pohl and co-workers from Iowa State University (J. Am. PMID:34645436 Fmoc-L-Val-OH supplier Chem. Soc. 2004, 126, 13188.DOI: 10.1021/ja045522j)starting from a protected D-glucose. Microwave-enhanced iodination of the selectively protected glucose precursor took place in one min in toluene at 60 °C in the presence of iodine and triphenylphosphine. Subsequent base-promoted elimination of hydrogen iodide in the presence of DBU required 30 min (DMF, 80 °C), and the key Ferrier rearrangement induced by palladium dichloride was also completed within 5 min of microwave irradiation. The development of this series of microwave-assisted transformations significantly shortened the time to form the core carbocyclic structure from a protected glucose.
Negishi Couplings
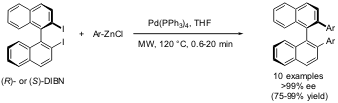
An application of high-speedNegishi coupling methodology for the preparation of enantiopure 2,2′-diarylated 1,1′-binaphthyls was reported by Putala and co-workers from the Slovak republic (Chem. Commun. 2004, advance article,DOI: 10.1039/b410185e). Reaction times as short as 40 seconds in some cases were sufficient to achieve complete conversions in the stereoconservative Negishi coupling of commercially available 2,2′-diiodo-1,1′-binaphthyl (DIBN) with arylzinc chlorides. The catalyst loading for typical runs was 5 mol% of Pd(PPh3)4, but could be lowered to 0.5 mol% in some instances without appreciable reduction of coupling efficiency. The formed enantiopure 2,2′-diarylated 1,1′-binaphthyls are of interest in material sciences application. In the same article, the corresponding Negishi alkynations using zinc phenylacetylide and zinc trimethylsilylacetylide were also described.
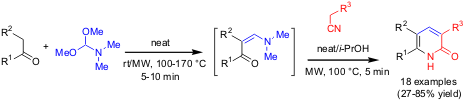
Multicomponent Synthesis of 2-Pyridones
We have described (Tetrahedron 2004, 60, 8633.DOI: 10.1016/j.tet.2004.05.100)a multicomponent, one-pot, two-step pathway to 3,5,6-substituted 2-pyridones. In the first step equimolar mixtures of a CH-acidic carbonyl compound andN,N-dimethylformamide dimethyl acetal (DMFDMA) were reacted to form the corresponding enamines, either at room temperature or under microwave conditions. After addition of a methylene active nitrile (1 equiv), 2-propanol as solvent and catalytic amounts of piperidine base, the reaction mixture was heated to 100 °C for 5 minutes under microwave conditions. In most cases, the desired heterocyclic product precipitated directly after cooling of the reaction mixture and could be collected by filtration.