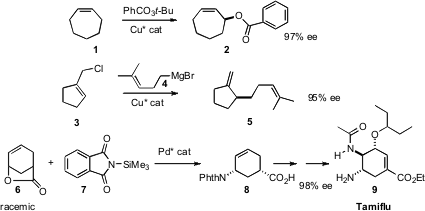
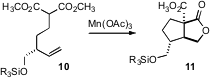Both 1 and 3 are inexpensive prochiral starting materials. Tae-Jong Kim of Kyungpook National University devised (Organomet. 2008, 27, 1026.DOI: 10.1021/om700935m) a chiral Cu catalyst that efficiently converted 1 (other ring sizes worked as well) to the enantiomerically pure ester 2. Alexandre Alexakis of the University of Geneva found (Adv. Synth. Catal. 2008, 350, 1090.DOI: 10.1002/adsc.200800086)a chiral Cu catalyst that mediated the enantioselective coupling of 3 withGrignard reagents such as 4. The π-allyl Pd complex derived from 6 is also prochiral. Barry M. Trost of Stanford University showed (Angew. Chem. PMID:23880095 Int. Ed. 2008, 47, 3759.DOI: 10.1002/anie.200800282)that with appropriate ligand substitution, coupling with the phthalimide 7 proceeded to give 8, readily convertible to (-)-oseltamivir (Tamiflu) (9), in high ee.
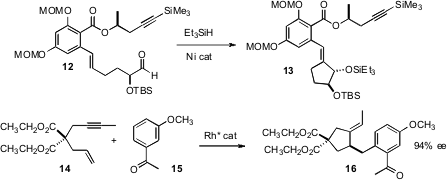
Jonathan W. Azido-PEG1 Formula Burton of the University of Oxford found (Chem Commun. Price of [Rh(COD)2]BF4 2008, 2559. DOI: 10.1039/b802473a)that Mn(OAc)3-mediated cyclization of 10 delivered the lactone 12 with high diastereocontrol. John Montgomery of the University of Michigan observed (Org. Lett. 2008, 10, 811.DOI: 10.1021/ol702961v)that the Ni-catalyzed cyclization of 12 also proceeded with high diastereocontrol. Ken Tanaka of the Tokyo University of Agriculture and Technology combined (Angew. Chem. Int. Ed. 2008, 47, 1312.DOI: 10.1002/anie.200704758)Rh-catalyzed ene-yne cyclization of 14 with catalytic ortho C-H functionalization, leading to 16 in high ee.
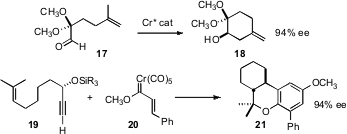
Eric N. Jacobsen of Harvard University designed (Angew. Chem. Int. Ed. 2008, 47, 1469.DOI: 10.1002/anie.200704439)a chiral Cr catalyst for the intramolecular carbonyl ene reaction, that converted 17 to 18 in high ee. Using a stoichiometric prochiral Cr carbene complex 20 and the enantiomerically-pure secondary propargylic ether 19, Willam D. Wulff of Michigan State University prepared (J. Am. Chem. Soc. 2008, 130, 2898. DOI: 10.1021/ja077579m)a facially-selective Cr-complexedo-quinone methide intermediate, that cyclized to 21 with high ee.
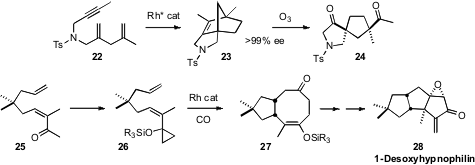
A variety of methods have been put forward for the transition metal-mediated construction of polycarbocyclic systems. One of the more powerful is the enantioselective Rh-catalyzed stitching of the simple substrate 22 into the tricycle 23 devised (J. Am. Chem. Soc. 2008, 130, 3451.DOI: 10.1021/ja0762083)by Takanori Shibata of Waseda University. Inter alia, ozonolysis of 23 delivered the cyclopentane24 containing two all-carbon quaternary centers. Zhi-Xiang Yu of Peking University has devised both carbonylative (J. Am. Chem. Soc. 2008, 130, 4421.DOI: 10.1021/ja7100449), illustrated, and non-carbonylative (J. Am. Chem. Soc. 2008, 130, 7178.DOI: 10.1021/ja8008715)Rh-catalyzed cylization of alkenyl cyclopropanes such as 26. Intramolecular aldol condensation followed by further functionalization converted 27 into 1-desoxyhypnophilin (28).