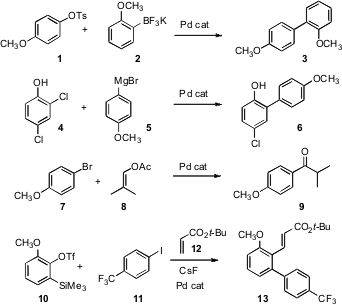
Tosylates are among the least expensive, but also among the least reactive toward Pd(0) oxidative addition, of aryl sulfonates. Jie Wu of Fudan University has now devised conditions (J. Org. Chem. 2007, 72, 9346.DOI: 10.1021/jo7019064)for the Pd-catalyzed coupling of aryl tosylates such as 1 with arene trifluoroborates. Kei Manabe of RIKEN has found (Org. Lett. PMID:23439434 2007,9, 5593. DOI: 10.1021/ol702646s)that an ortho OH activates an adjacent Cl for Pd-mediated coupling, allowing the conversion of 4 to 6. Philippe Uriac and Pierre van de Weghe of the Université de Rennes I have developed (Org. BuySalcaprozate (sodium) Lett. 2007,9, 3623. DOI: 10.1021/ol7015065)conditions for the catalytic acylation of aryl halides with alkenyl acetates such as 8.
Multi-component coupling lends itself well to diversity-oriented synthesis. As illustrated by the combination of 10 with 11 and 12 to give 13 reported (Org. Lett. 3-Chloropropionaldehydediethylacetal Price 2007, 9, 5589.DOI: 10.1021/ol702584t)by Michael F. Greaney of the University of Edinburgh, benzynes can do double addition with high regiocontrol. For other recent references to unsymmetrical double additions to arynes, see Angew. Chem. Int. Ed. 2007, 46, 5921, DOI: 10.1002/anie.200701063;Chem. Commun. 2007, 2405,DOI: 10.1039/b701581jand J. Am. Chem. Soc. 2006, 128, 14042,DOI: 10.1021/ja0662671.
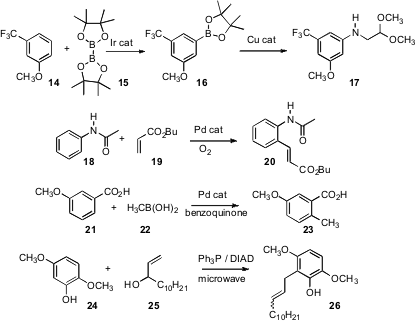
C-H functionalization of arenes is of increasing importance. John F. Hartwig of the University of Illinois has described (Org. Lett. 2007, 9, 757, DOI: 10.1021/ol062903o 761,DOI: 10.1021/ol062902w)improved conditions for Ir-catalyzed meta borylation, and conditions for further coupling of the initial borate 16 to give amines such as 17. Lei Liu and Qing-Xiang Guo of the University of Science and Technology, Hefei have found (Tetrahedron Lett. 2007, 48, 5449.DOI: 10.1016/j.tetlet.2007.06.001)that oxygen can be used as the stoichiometric oxidant in the Pd-catalyzed functionalization of H’s ortho to anilides. Two other research groups (J. Am. Chem. Soc. 2007, 129, 6066,DOI: 10.1021/ja070767s;Angew. Chem. Int. Ed. 2007, 46, 5554,DOI: 10.1002/anie.200700590;J. Org. Chem. 2007, 72, 7720,DOI: 10.1021/jo701387m)reported advances in this area. In a close competition, Jin-Quan Yu, now at Scripps/La Jolla (J. Am. Chem. Soc. 2007, 129, 3510.DOI: 10.1021/ja0701614)and Olafs Daugulis of the University of Houston (J. Am. Chem. Soc. 2007, 129, 9879. DOI: 10.1021/ja071845e)both reported that a carboxyl group can activate an ortho H for direct functionalization. Note that metalation of 21 would be expected to proceed ortho to the methoxy group.
There are other strategies for directly functionalizing ortho positions. Christopher J. Moody of the University of Nottingham has found (J. Org. Chem. 2007, 72, 10298.DOI: 10.1021/jo702101w)that phenols such as 24 can be coupled with secondary allylic alcohols to give, afterClaisen rearrangement, the alkylated product 26.
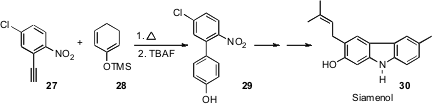
For highly-substituted benzene derivatives, it is sometimes more efficient to build the aromatic ring. Rich G. Carter of Oregon State University, in the course of a synthesis of siamenol 30, developed (J. Org. Chem. 2007, 72, 9857.DOI: 10.1021/jo070740r)the Diels-Alder addition of aryl alkynes such as 27 to enol derivatives such as 28 to give the biphenyl 29.