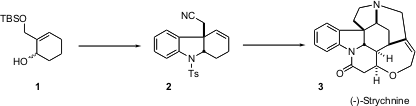
The total synthesis of (-)-strychnine 3 reported (J. Am. Chem. Buy(S)-Tetrahydrofuran-3-carboxylic acid Soc. 2003, 125, 9801.DOI: 10.1021/ja029382u)by Miwako Mori of Hokkaido University is a tour de force of selective organopalladium couplings.
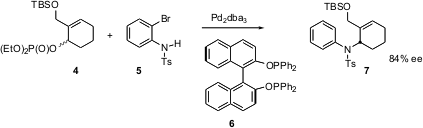
The absolute configuration of the final product was established at the outset, by Pd-catalyzed de-racemizing coupling of 4 with the o-bromoaniline derivative 5. PMID:24578169 Using the inexpensive Binol-derived ligand (S)-BINAPO 6 , a model coupling was carried out on several cyclohexenol derivatives having different one-carbon substituents at C-2. The best ee’s were observed with the silyloxymethyl group. 1141886-37-4 Order Several alcohol derivatives were then tried, and it was found that the allylic phosphate gave the best rates and ee’s. Using the optimized4, the coupling with 5 to give 7 proceeded in 84% ee.
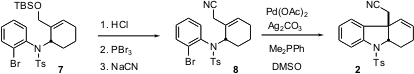
The sidechain of 7 was extended by one carbon, to give the nitrile 8. A second organopalladium step then was used to cyclize 8 to 2. Using Ag2CO3 as the base suppressed unwanted alkene migration. The reaction ran more slowly in DMSO than in DMF, but byproduct formation was suppressed.
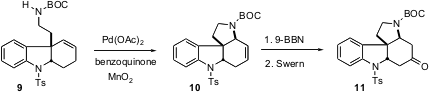
The crystalline nitrile 2 (99% ee from EtOH) was reduced and protected to give the carbamate9, setting the stage for another Pd-catalyzed ring-forming step. Allylic oxidation of 9 gave the enone only in unacceptably low yield. Pd-mediated cyclization, by contrast, proceeded efficiently to give the alkene10. Hydroboration followed by oxidation then gave the ketone 11, a useful intermediate for the construction of a variety of Strychnos alkaloids.
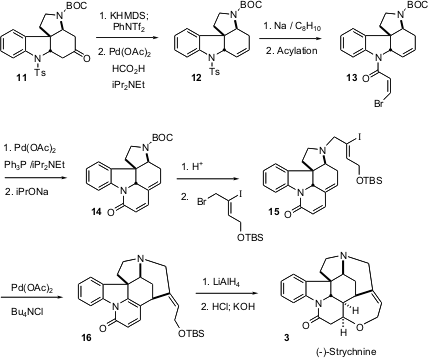
For strychnine 3, the ketone 11 was converted to the alkene 12 by reduction of the enol triflate derived from the more stable enolate. Deprotection and acylation gave13, which was cyclized with Pd to give, after equilibration, the diene 14. Alkylation, to give15, followed by Pd-mediated cyclization then gave 16, which was reduced and cyclized to (-)-strychnine3.