The synthesis of 2-arylindoles 3 via tandem radical cyclization of the corresponding acrylates 1 and subsequent oxidation was reported by the group of Oliver Reiser from the Universtiy of Regensburg, Germany (Synthesis2008, 2191. 623583-09-5 site DOI: 10.1055/s-2008-1067154). In the first step of the synthesis, acrylates 1 are transformed to 2-arylindolines 2 by a tributyltin hydride mediated radical cyclization involving a 1,6-H transfer followed by a 5-exo ring closure under open vessel microwave conditions. For the aromatization step, DDQ proved to be the oxidation reagent of choice and 2-arylindoles were obtained in good yields. For both procedures, higher yields and reduced reaction times could be achieved when applying microwave heating.

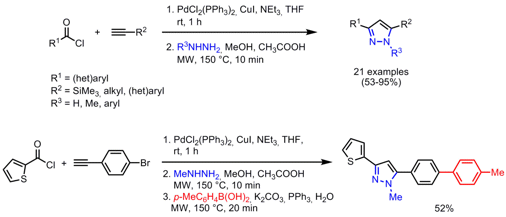
One-Pot Three-Component Synthesis of 1,3,5-Trisubstituted Pyrazoles
Thomas Müller and Benjamin Willy from Heinrich-Heine University, Düsseldorf, have developed a one-pot protocol for the synthesis of highly fluorescent 3,5- and 1,3,5-substitued pyrazoles (Eur. PMID:24238415 J. Org. Price of 494767-19-0 Chem. 2008, 4157. DOI: 10.1002/ejoc.200800444). In the first sequence, alkynones are obtained by a Sonogashira coupling of acyl chlorides with terminal alkynes at room temperature. Subsequent addition of hydrazines, MeOH and acetic acid under microwave heating to 150 °C for 10 min furnished the pyrazoles regioselectively + depending on the hydrazine substituents R3 – via a Michael addition/cyclocondensation step. Further scaffold decoration for one example was accomplished in a one-pot four-component fashion by performing an additional Suzuki coupling step employing the catalyst system from step one, again under microwave heating giving a biphenyl substituted pyrazole.
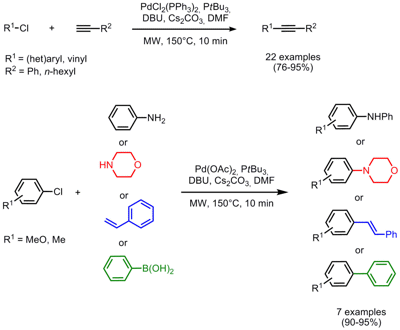
Cross-Coupling Reactions with Aryl Chlorides
Hong Liu and co-workers from the Chinese Academy of Sciences, Shanghai, have successfully performed Sonogashira cross-couplings of terminal alkynes with a variety of aryl chlorides (J. Org. Chem. 2008, 73, 6037. DOI: 10.1021/jo800994f). This copper-free method proved to be very general and also both sterically hindered and electron-rich aryl chlorides can be coupled very efficiently in addition to electron-neutral and -deficient ones giving the products in high yield and short reaction times. Furthermore, this protocol can be applied for other Pd-catalyzed C-C and C-N bond forming reactions by switching from PdCl2(PPh3)2 to Pd(OAc)2 as catalyst. Buchwald-Hartwig aminations, Suzuki- and Heck couplings were conducted delivering the coupled products in excellent yields (90-95%).
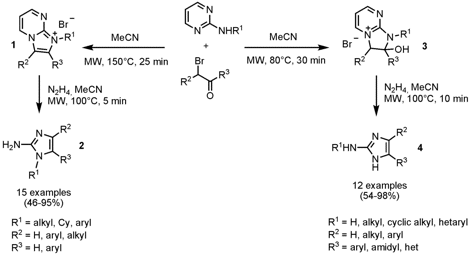
Synthesis of 2-Aminoimidazoles from 2-Aminopyrimidines
By applying one-pot two-step procedures, Erik Van der Eycken and Denis Ermolat´ev from the University of Leuven, Belgium, prepared 1-substituted and unsubstituted 2-aminoimidazoles 2 and 4 (J. Org. Chem. 2008, 73, Asap.DOI: 10.1021/jo8008758). By a cyclocondensation sequence of 2-aminopyrimidines with α-bromo carbonyl compounds at 150 °C imidazopyrimidinium salts 1 are obtained as intermediates which undergo cleavage using hydrazine hydrate furnishing 1-substituted 2-aminoimidazoles 2. However, is the cyclocondensation step performed at 80 °C, 2-hydroxy-2,3-dihydroimidazopyrimidinium salts 3 are formed that can be subsequently cleaved with hydrazine hydrate to give the 1-unsubstituted 2-aminoimidazoles 4. The cleavage step for the formation of products 4 is proposed to proceed via a Dimroth-type rearrangement with an in situ generation of 2-amino-5-hydroxyimidazolidine.
.