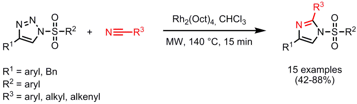
The groups of Vladimir Gevorgyan and Valery Fokin from the University of Illinios and The Scripps Research Institute have reported on the synthesis of imidazoles via the transannulation of 1-sulfonyl triazoles with nitriles using rhodium(II) octanoate as catalyst (J. PMID:24576999 Am. Chem. Soc. 2008, 130, 14972. 849805-25-0 In stock DOI: 10.1021/ja805079v). Formula of 4-(Diethylphosphinyl)benzenamine When applying microwave heating no inert atmosphere is necessary compared to performing the reactions under conventional heating at 80 °C where – in addition – longer reaction times of 12-24 h are needed. Furthermore, the authors were successful in combining the synthesis of 1-sulfonyl triazoles – via the Cu(I)-catalyzed cycloaddition of sulfonyl azides with alkynes – and the transannulation in a one-pot two-step sequence.
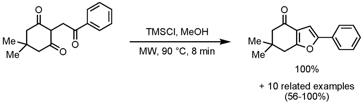
Synthesis of 4-Keto-4,5,6,7-tetrahydrobenzofurans
Alain Wagner, Rachid Baati and coworkers from Université Louis Pasteur, France, have disclosed the synthesis of 4-keto-4,5,6,7-tetrahydrobenzofurans via an intramolecular cyclization of triketones (Tetrahedron Lett. 2008,49, ASAP.DOI: 10.1016/j.tetlet.2008.10.139). Key to the success was the employment of 4 equiv. TMSCl in MeOH as a source of anhydrous HCl. In general excellent product yields are obtained, only for 1,3-dicarbonyls with an amide functionality a lower yield (56%) of the corresponding oxazole is achieved. Whereas acyclic and 7-membered triketones furnished the products in high yields, 5-membered triketones were completely unreactive and only starting material was recovered.
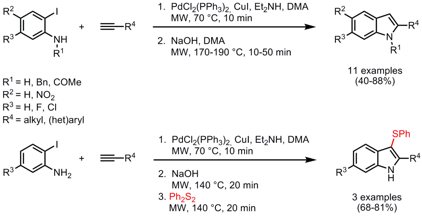
One-Pot Sonogashira Coupling-NaOH-Mediated Cyclization
The synthesis of 2-substituted indoles by a one-pot two-step sequence starting from o-iodoanilines and terminal alkynes was described by the group of Roberto Sanz from Universidad de Burgos, Spain (Synlett 2008, 3006. DOI: 10.1055/s-0028-1083624). The first step includes a Sonogashira coupling leading to o-alkynylanilines which were subsequently cyclized under mediation of NaOH to the 2-substituted indoles. Both steps were performed under microwave heating which had the advantage of employing less NaOH (4 vs. 10 equiv.) and reduced reaction times compared to conventional heating (1-2 h at rt for step 1 and up to 6 h at 140 °C for step 2). In addition, an arylthio group can be selectively introduced at the C-3 position in a one-pot three-step procedure.
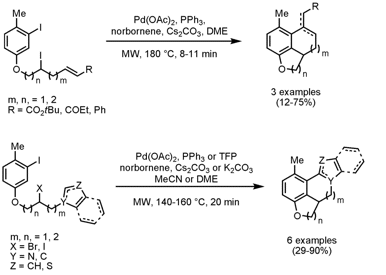
o-Alkylation of Aryl Iodides with Secondary Alkyl Halides
Mark Lautens and coworkers from the University of Toronto have developed Pd-catalyzed norbornene-mediated intramolecular o-alkylations of aryl iodides with secondary alkyl iodides and bromides for the synthesis of polycylic heterocycles (J. Org. Chem. 2008, 73, ASAP.DOI: 10.1021/jo802180h). These alkylations proceed in a domino process with subsequent termination steps such as Heck reactions, direct arylations or Buchwald-Hartwig aminations in one-pot forming new C-C or C-N bonds. In the domino intramolecular o-alkylation/Heck reaction tricyclic heterocycles are formed with separable exo- and endocyclic double bond isomers for products with an O-containing 5-membered ring. Pyrrole, indole and thiophene containing substrates undergo an intramolecular o-alkylation/direct arylation sequence delivering tetra- and pentacyclic heterocycles. In addition, the reaction sequence occurs in a stereospecific manner which was validated by the use of enantioenriched substrates where a minimal loss of ee was obtained for phenol tethered alkyl iodides. However, secondary alkyl bromides gave the product in more dimished ee.
.