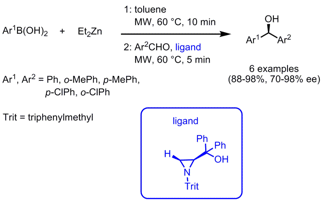
The groups of Antonio Braga und Ludger Wessjohann from Universidade Federal de Santa Maria, Brazil, and the Leibniz Institute of Plant Biochemistry, Germany, have reported on the arylation of aromatic aldehydes via arylzinc addition using an aziridine-based ligand (J. Buy1272758-17-4 Org. Chem. PMID:23710097 2008, 73, 2879. DOI: 10.1021/jo702413n). Employing microwave heating, the reactive arylzinc species are generated from aryl boronic acids and Et2Zn in only 10 min followed by the addition of the aldehyde and ligand and further microwave heating for 5 min. 2252403-85-1 Chemscene The reaction time can be reduced from 1 day conventionally to only 15 min under microwave conditions. A key feature of this method is that both enantiomers of the product can be obtained selectively using only one enantiomer of the ligand by interchanging the functional groups of the boronic acids and aldehydes.
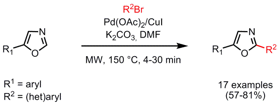
Ligandless Direct Arylation of Oxazoles
The Pd-catalyzed, Cu-mediated C-2 direct arylation of 5-oxazoles with aryl bromides was disclosed by Sandrine Piguel and co-workers from the Institut Curie/CNRS, France (J. Org. Chem. 2008, 73, 3278. DOI: 10.1021/jo7027135). This method shows a high functional group tolerance and in addition the reaction times can be reduced from hours to minutes when employing microwave heating. In particular, electron-poor oxazoles need only very short reaction times of 4-8 min to give the 2,5-diaryloxazoles in higher yields.
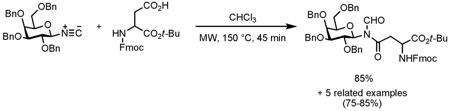
Reaction of Isonitriles with Carboxylic Acids
Samuel Danishefsky and Xuechen Li from the Sloan-Kettering Institute for Cancer Research, New York, have investigated the reaction of isonitriles with carboxylic acids to form N-formyl amides (J. Am. Chem. Soc. 2008, 130, ASAP. DOI: 10.1021/ja800612r). The reactions proceed well under microwave irradiation conditions at 150 °C for 30-45 min (depending on the substrates) possibly through a 1,3-O→N-acyl transfer whereas at room temperature no reaction could be detected. When glycosyl-linked isonitriles are reacted with aspartate, the reactions occur in an anomerically specific way to the corresponding α- and β- N-linked glycosyl amino acids. Further transformations of the N-formyl amides to different amide types are described as well.
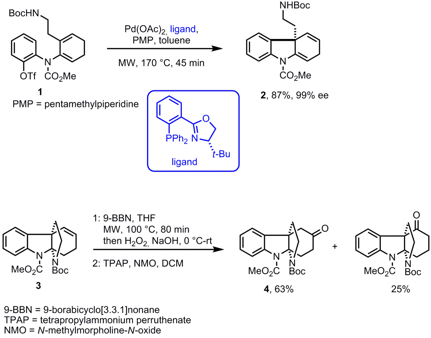
Total Synthesis of (+)-Minfiensine
In the course of the synthesis of the Strychnos alkaloid minfiensine Larry Overman and his group from the University of California, Irvine, have conducted two reaction sequences with microwave irradiation (J. Am. Chem. Soc. 2008, 130, 5368. DOI: 10.1021/ja800163v). As a key step, the intramolecular asymmetricHeck reaction of precursor 1 was performed at 170°C within 45 min and afforded the cyclized product 2 in high yield and excellent 99% ee. Due to the short reaction times the catalyst loading could be decreased from 20 mol% to 10 mol%. The employed PHOX ligand proved to be optimal in order to prevent double bond migration. In a second-generation total synthesis of (+)-minfiensine, the hydroboration of scaffold 3 proceeded under microwave heating with9-BBN in THF at 100 °C whereas in refluxing THF no reaction took place. After oxidation of the crude product with TPAP/NMO the desired ketone 4 was obtained as major product in 63% yield. (+)-Minfiensine was synthesized in 15 steps and 6.5% overall yield with the second-generation protocol.
.