Currently, glycosylation is still a very challenging reaction due to the delicate balance between the reactivity and stereoselectivity.
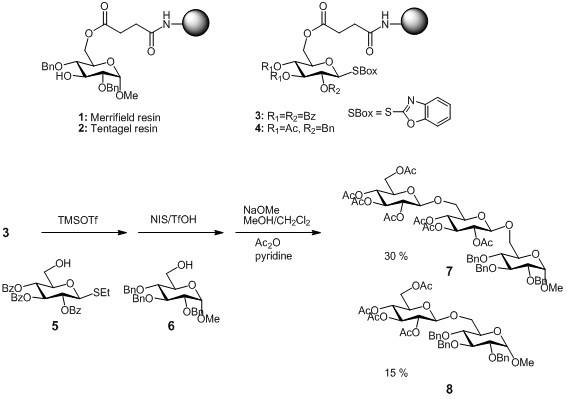
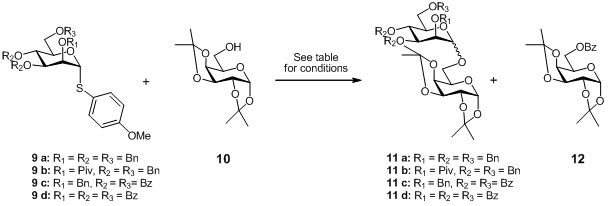
Solid phase synthesis has been recently described as an efficient alternative to solution synthesis, in some cases giving better yields and overcoming stereochemical problems. PMID:28322188 M. Parlato et al. reported very rapid and stereoselective solution-phase glycosylations using S-benzoxazolyl (SBox) and S-thiazolinyl (STaz) glycosides, under mild reaction conditions. This group also demonstrated that these derivatives were suitable for both the glycosyl acceptor-bound and glycosyl donor-bound strategies commonly employed in resin-supported oligosaccharide synthesis. (J. Buy145100-51-2 Org. Chem. 2008, 73, 1716. DOI: 10.1021/jo701902f)
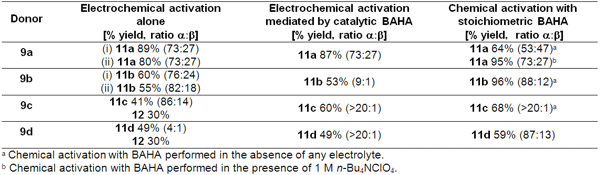
Fairbanks and co-workers (J. Phys. Org. DBCO-PEG4-NHS ester structure Chem. 2008, 21, 516. DOI: 10.1002/poc.1388)described the electrochemical glycosylation of thioglycoside donors, alone or in the presence of a catalytic amount of the chemical mediator tris(4-bromophenyl)aminium hexachloroantimonate (BAHA).
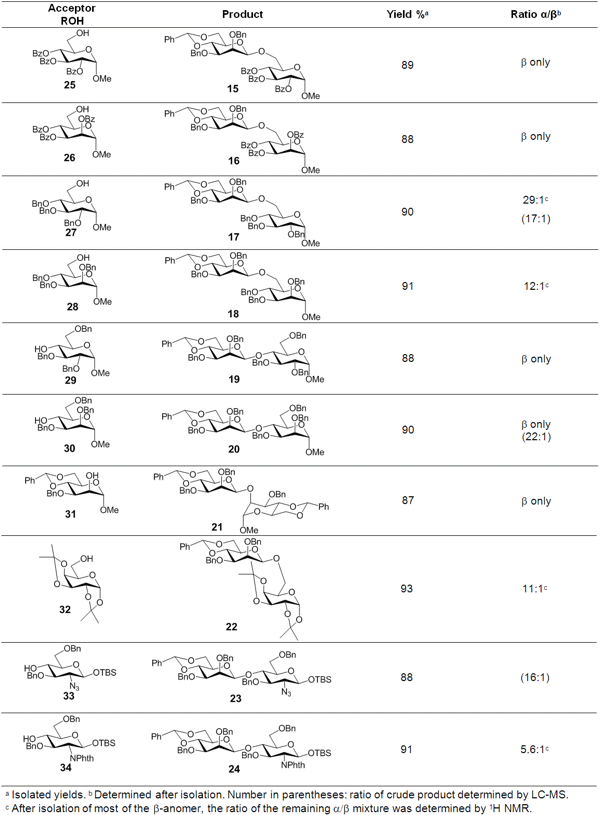
Kwan Soo Kim et al. (J. Am. Chem. Soc. 2008, 130, 8537. DOI: 10.1021/ja710935z)reported a new stereoselective direct glycosylation with anomeric hydroxy sugars, where the anomeric hydroxy donor is treated sequentially with phthalic anhydride, triflic anhydride, and an acceptor alcohol in the presence of an appropriate base, in a one-pot procedure without isolation.
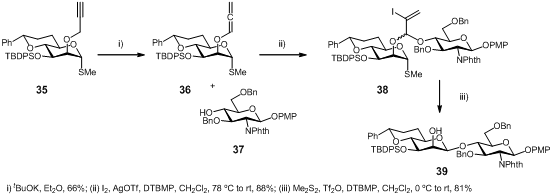
Intramolecular aglycon delivery (IAD), which is a particular type of intramolecular glycosylation method, appeared as a solution to the ‘1,2-cis glycoside problem’, wherein the glycosyl acceptor is temporarily appended to the 2-hydroxyl group of a glycosyl donor through a short linker. In this work, E. Attolino et al. (Tetrahedron: Asymmetry 2007, 18, 1721. DOI: 10.1016/j.tetasy.2007.06.026)describe the development of propargyl mediated IAD for the synthesis of the key Man(β-1,4)GlcNAc linkage of N-glycan oligosaccharides.
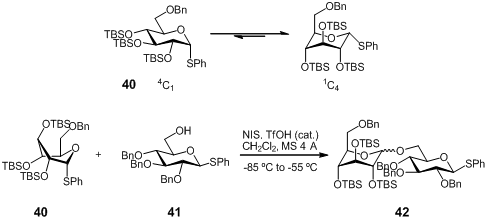
M. Bols and co-workers (J. Am. Chem. Soc. 2007, 129, 9222. DOI: 10.1021/ja071955l)investigated the reactivity of glycosyl, rhamnosyl, mannosyl, and galactosyl donors protected on the 2-, 3-, and 4-OH groups with bulky silyl protective groups (tert-butyldimethylsilyl, TBS), and carried out a number of glycosylation reactions with them. The enhanced reactivity is explained by the stereoelectronic effects associated with the conformational change induced by the silylation, with the conclusion that they are considerably more reactive than benzylated (armed) donors.