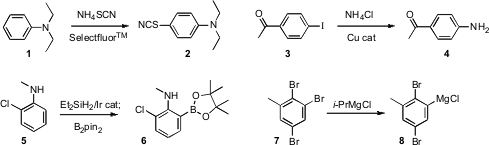
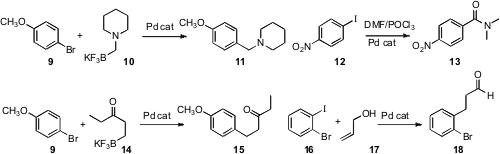
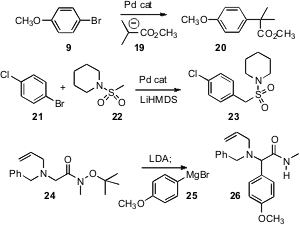
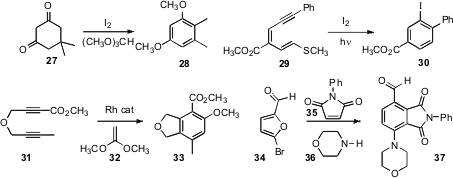
Several new methods have been put forward for the functionalization of
benzene derivatives. J. S. Yadav of the Indian Institute of Chemical Technology,
Hyderabad has devised (Chem. Lett. 2008, 37, 652.
DOI: 10.1246/cl.2008.652)
a procedure for direct thiocyanation, converting 1 into 2. Sukbok
Chang of KAIST has established (Chem. Commun. 2008, 3052.
DOI: 10.1039/b804637a)
that both NH4Cl and aqueous NH3 could be used to directly
aminate an aryl iodide such as 3. John F. PMID:23937941 Hartwig of the University of
Illinois has developed (J. Am. Chem. Soc. 2008, 130, 7534.
DOI: 10.1021/ja8015878)
a protocol for the directed borylation of anilines such as 5 and of
phenols, based on a transient silylation. Karsten Menzel of Merck West Point (Tetrahedron
Lett. 2008, 49, 415.
DOI: 10.1016/j.tetlet.2007.11.124)
has observed selective exchange of
tribromobenzene derivatives such as 7, with the direction of the
selectivity being controlled by the fourth substituent on the benzene.
Gary A. Molander of the University of Pennsylvania has extensively developed
the stable, readily prepared trifluoroborates, exemplified by 10 (J. 1334146-82-5 Price
Org. 2387561-40-0 manufacturer Chem. 2008, 73, 2052.
DOI: 10.1021/jo800183q)
and 14 (Org. Lett.
2008, 10, 1795.
DOI: 10.1021/ol800357c)
as partners for Suzuki-Miyaura coupling. The conversion of 9 to 10 is complementary to aminocarbonylation,
exemplified by the conversion of 12 to 13 reported (Tetrahedron
Lett. 2008, 49, 2221.
DOI: 10.1016/j.tetlet.2008.02.049)
by Bhalchandra M. Bhanage of the
Institute of Chemical Technology, University of Mumbai. The coupling of 9
with 14 is complementary to the long-known
Heck coupling of an aryl
halide such as 16 with an allylic alcohol, as illustrated by the
preparation of 18 described (Tetrahedron Lett. 2008, 49,
3279.
DOI: 10.1016/j.tetlet.2008.03.067)
by Martin E. Maier of the Universität Tübingen.
Professor Hartwig has also (Org. Lett. 2008, 10, 1545,
DOI: 10.1021/ol80025781549,
DOI: 10.1021/ol800258u)
optimized conditions for the Pd-catalyzed arylation of ester
enolates such as 19. Gang Zhou of Schering-Plough, Kenilworth, NJ has
developed (Org. Lett. 2008, 10, 2517.
DOI: 10.1021/ol800785g)
a related
transformation, the arylation of deprotonated sulfonamides. Peter Somfai of the
Royal Institute of Technology, Stockholm has established (Angew. Chem. Int.
Ed. 2008, 47, 1907.
DOI: 10.1002/anie.200704689)
a complementary procedure,
base-mediated elimination of t-butoxide from 24, followed by
1,2-addition of an aryl or heteroaryl
Grignard reagent.
For some substitution patterns, it is more efficient to construct the benzene
ring. Professor Yadav observed (Tetrahedron Lett. 2008, 49,
3810.
DOI: 10.1016/j.tetlet.2008.03.151)
that 27 was aromatized to 28. Katsuyuki Ogura of Chiba
University showed (J. Org. Chem. 2008, 73, 1726.
DOI: 10.1021/jo701976q)
that
29 cyclized to 30. Ken Tanaka of the Tokyo University of
Agriculture and Technology established (Org. Lett. 2008, 10,
2537.
DOI: 10.1021/ol800813g)
that enol ethers or enol acetates could take the place of an alkyne
partner in a trimerization reaction, as illustrated by the regioselective
cycloaromatization of 31 to 33. For a related study, see
Tetrahedron Lett. 2008, 49, 445,
DOI: 10.1016/j.tetlet.2007.11.103.
Sylvain Marque and Damien Prim of the Université de Versailles-Saint-Quentin-en-Yvelines have
found (J. Org. Chem. 2008, 73, 2191.
DOI: 10.1021/jo7024916)
that activation of 34 by condensation with a secondary amine set the stage for
Diels-Alder cycloaddition and aromatization, leading to 37. For related
studies, see Org. Lett. 2008, 10, 233,
DOI: 10.1021/ol702614b and
Tetrahedron Lett. 2008, 49, 219,
DOI: 10.1016/j.tetlet.2007.11.085.