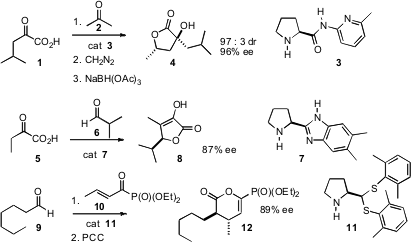
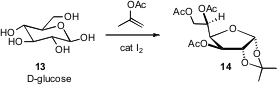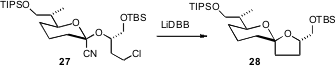Developments in organocatalysis have turned toward the enantioselective construction of lactones. Buy387859-70-3 Shi-Wei Luo and Liu-Zhu Gong of the University of Science and Technology of China have found (J. Org. Chem. 2007, 71, 9905. DOI: 10.1021/jo701868t)that catalyzed addition of acetone to an α-hydroxy acid1 proceeded with high ee. Methyl (S)-2-(Boc-amino)-4-bromobutyrate Chemscene Esterification of the addition product followed by reduction and acid work-up delivered the lactone 4 with high dr and ee. In a complementary approach, Jean-Marc Vincent and Yannick Landais of the University Bourdeaux-1 showed (Chem. Commun. 2007, 4782.DOI: 10.1039/b711192d)that catalyzed condensation of an aldehyde with an α-hydroxy acid 5 delivered the tetronic acid 8 in high ee. It may be that 8 could also be reduced with useful selectivity. Cong-Gui Zhao of the University of Texas, San Antonio has devised conditions (Org. Lett. 2007, 9, 2745. DOI: 10.1021/ol071097y)for the condensation of the keto phosphonates such as 10 with aldehydes to give, after oxidation, the δ-lactone 12. PMID:24458656
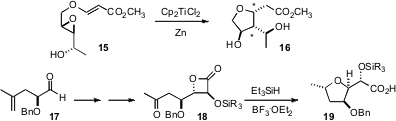
Carbohydrates such as glucose 13 are inexpensive, molecularly-complex starting materials. Subhash Chandra Taneja of the Indian Institute of Integrative Medicine, Jammu Tawi, has found conditions (J. Org. Chem. 2007, 72, 8965. DOI: 10.1021/jo070363i)for the single-step I2-catalyzed transformation of 13 to 14, in which each of the alcohols have been differentiated. In a complementary approach described (Tetrahedron Lett. 2007, 48, 6389.DOI: 10.1016/j.tetlet.2007.06.139)by Tushar Kanti Chakraborty of the Indian Institute of Chemical Technology, Hyderabad, Ti-mediated reduction of 15 was shown to be highly diastereoselective, setting the two new stereogenic centers (marked by *) in 16. Building on work by Mead, Daniel Romo of Texas A&M has shown (J. Org. Chem. 2007, 72, 9053.DOI: 10.1021/jo7014246)that reductive cyclization of 18 also proceeded with high diastereocontrol, to give 19.
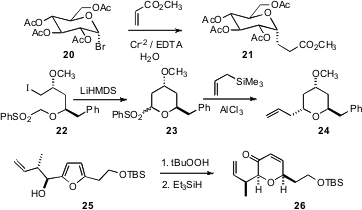
As illustrated by the conversion of 20 to 21 reported (Tetrahedron Lett. 2007, 48, 7351.DOI: 10.1016/j.tetlet.2007.08.019)by Zsuzsa Juhász and László Somsák of the University of Debrecen, six-membered ring cyclic ethers can also be formed from carbohydrate precursors. Richard E. Taylor of the University of Notre Dame has taken advantage (Angew. Chem. Int. Ed. 2007, 46, 6874.DOI: 10.1002/anie.200702018)of the “chemical chameleon” nature of a sulfone, using it both the stablilize the anion for intramolecular alkylation, to form 23, and as a leaving group, leading to 24. Andrew J. Phillips of the University of Colorado has oxidized (Org. Lett. 2007, 9, 5299.DOI: 10.1021/ol702559e), the enantiomerically-pure furan 25 to give, after reduction, the ring-expandedcyclic ether 26.
Spiroketals such as 28 are usual prepared under equilibrating conditions, leading to the most stable diastereromer. Yet, there are spiroketal natural products that are the less stable diastereomer, prepared under kinetic conditions. Scott V. Rychnovsky of the University of California, Irvine has found (Org. Lett. 2007, 9, 711.DOI: 10.1021/ol0630447)that the reductive cyclization of a halo nitrile 27 can kinetically establish the less-stable spiroketal 28.