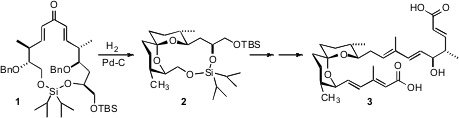
Often, 6,6-spiroketals such as Spirofungin A (3) have a strong anomeric bias. Spirofungin A does not, as the epimer favored by double anomeric stabilization suffers from destabilizing steric interactions. Tetrabutylammonium periodate Order In his synthesis of 3, Sergey A. Kozmin of the University of Chicago took advantage (Angew. Chem. Int. 9-Oxo-9H-fluorene-4-carboxylic acid Chemscene Ed. PMID:24381199 2007, 46, 8854.DOI: 10.1002/anie.200702440)of the normally-destablizing spatial proximity of the two alkyl branches of 3, joining them with a siloxy linker to assure the anomeric preference of the spiroketal. The assembly of 1 showcased the power of asymmetric crotylation, and of Professor Kozmin’s linchpincyclopropenone ketalcross metathesis.
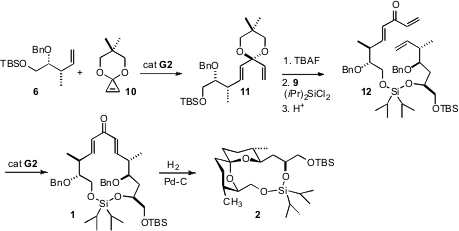
To achieve the syn relative (and absolute) configuration of 6, commercial cis-2-butene was metalated, then condensed with the Brown (+)-MeOB(Ipc)2 auxiliary. The accompanying Supporting Information, accessible via the online HTML version of the journal article, includes a succinct but detailed procedure for carrying out this homologation. For the anti relative (and absolute) configuration of 9, it is more convenient to use the tartrate 8 introduced by Roush.
Driven by the release of the ring strain inherent in 10, ring opening cross metathesis with 6 proceeded to give the 1:1 adduct 11 in near quantitative yield. The derived cross-linked silyl ether 12 underwent smooth ring-closing metathesis to the dienone 1.
On hydogenation, the now-flexible ring system could fold into the spiro ketal. With the primary and secondary alcohols bridged by the linking silyl ether, only one anomeric form, 2, of the spiro ketal was energetically accessible.
A remaining challenge was the stereocontrolled construction of the trisubstituted alkene. To this end, the aldehyde 13 was homologated to the dibromide 14. Pd-mediated coupling of the alkenyl stannane 15 with 14 was selective for the E bromide. The residual Z bromide was then coupled with Zn(CH3)2 to give 16. These steps, and the final steps to complete the construction of spirofungin A (3), could be carried out without exposure to equilibrating acid, so the carefully established spiro ketal configuration was maintained.