The construction of carbon-carbon bonds is fundamental to organic synthesis. PMID:23880095 Recently, three new methods have been reported, each of which has substantial potential for the synthesis of highly functionalized target molecules.
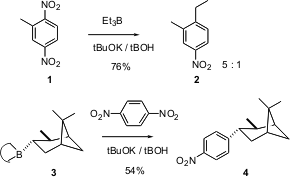
Shmaryahu Hoz of Bar-Ilan University reports (J. Org. Chem. 2003, 68, 4388.DOI: 10.1021/jo034218q)that alkyl boranes couple with dinitro aromatic rings such as 1 to give the alkylated aromatic, with loss of one of the nitro groups. 1394003-65-6 Data Sheet 1394041-21-4 site This reaction shows remarkable regioselectivity, as illustrated by the formation of2. Much more complex alkyl boranes participate also, as illustrated by the coupling of the 9-BBN derivative3. The reaction proceeded to give 4 as a single diastereomer.
The Heck reaction is well developed as a method for alkylating aromatics. Frank Glorius of the Max-Planck-Institute, Mülheim, reports (Tetrahedron Lett. 2003, 44, 5751.DOI: 10.1016/S0040-4039(03)01377-7)that chloroacetamides and bromoacetonitrile can also be activated by catalytic Pd to give the coupled products. This reaction works well with enol ethers, to give highly functionalized alkenes, but it also works well with a simple cyclic alkene.
The Pd-catalyzed homologation of aromatic rings does not necessarily require an aryl halide or other leaving group. The presence of a suitably disposed chelating group is sufficient to mediate metalation, followed by coupling. Masahiro Miura of Osaka University reports (J. Org. Chem. 2003, 68, 5236.DOI: 10.1021/jo0344034)that the tertiary alcohol 9 smoothly undergoes ortho palladation and coupling. The reaction can be limited to monocoupling, or extended to double homologation, to give10. Interestingly, the tertiary alcohol itself can also serve, with loss of acetone, as a precursor to the aryl Pd species.