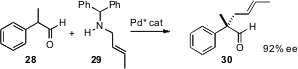The enantioselectivity of alkene reduction usually depends on the geometric purity of the alkene. Bruce H. Lipshutz of the University of California, Santa Barbara used (Org. Lett. 2007, 9, 4713.DOI: 10.1021/ol701999h)carboalumination of the alkyne 1 to prepare 2, which was selectively reduced to 3 in high ee. André B. Charette of the Université de Montréal reported (Angew. Chem. Int. Ed. 2007, 46, 5955.DOI: 10.1002/anie.200701367)a related reduction of unsaturated sulfones such as 4. Formula of Fmoc-Lys(Boc)-COCH2Cl Juan C. Carretero of the Universidad Autónoma de Madrid has developed (J. Thalidomide-4-OH structure Org. PMID:24065671 Chem. 2007,72, 9924. DOI: 10.1021/jo7016197)a complementary route to enantiomerically-enriched sulfones, by conjugate addition to the unsaturated pyridyl sulfone 6. Specifically for styrene derivatives, Hans-Günther Schmalz of the University of Cologne has shown (Org. Lett. 2007, 9, 3555. DOI: 10.1021/ol071228v)that the product 11 from enantioselective Rh-catalyzed hydroboration can be homologated to 13.
Conjugate addition of stabilized carbanions can also be carried out with high enantiocontrol. David A. Evans of Harvard University has described (J. Am. Chem. Soc. 2007, 129, 11583. DOI: 10.1021/ja0735913)the Ni-catalyzed addition of malonate 15 to nitroalkenes such as 14. Claudio Palomo of the Universidad de País Vasco (Angew. Chem. Int. Ed. 2007, 46, 8431. DOI: 10.1002/anie.200703261)and concurrently Yujiro Hayashi of the Tokyo University of Science (Org. Lett. 2007, 9, 5307. DOI: 10.1021/ol702545z)have developed organocatalytic protocols for the addition of nitromethane 18 to unsaturated aldehydes such as 17.
J. Michael Chong of the University of Waterloo has found (J. Am. Chem. Soc. 2007, 129, 4908. DOI: 10.1021/ja0713734)that the Binol-mediated enantioselective conjugate addition of alkenylboronic acids such as 22 required the additional activation of the aryl ketone. Shun-Jun Li of Suzhou University and Teck-Peng Loh of Nanyang Technical University have extended (J. Am. Chem. Soc. 2007, 129, 276. DOI: 10.1021/ja0666046)enantioselective conjugate to unsaturated esters such as 24 to more highly substituted Grignard reagents. Alexandre Alexakis of the University of Geneva has demonstrated (Tetrahedron Lett. 2007, 48, 7408. DOI: 10.1016/j.tetlet.2007.07.220)that Ac2O is compatible with Et2Zn conjugate addition conditions, leading directly to the trapped enolate 27. Selective cleavage of 27 can then be used to prepare acyclic derivatives.
Benjamin List of the Max-Planck-Institut, Mülheim has taken advantage (J. Am. Chem. Soc. 2007, 129, 11336. DOI: 10.1021/ja074678r)of the large-small differential of the substituents on the aldehyde 28. Enantioselective Pd-mediated rearrangement of the protonated enamine derived from condensation of28 with 29 delivered the aldehyde 30 in high ee.