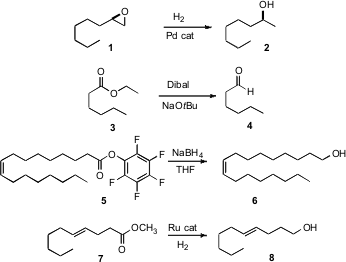
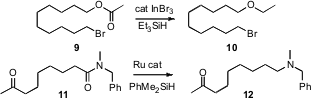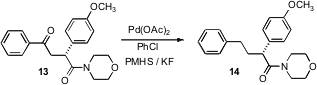Jaiwook Park of Pohang University of Science and Technology has developed (Org. Lett. 2007, 9, 3417.DOI: 10.1021/ol701456w)a procedure for the preparation of Pd-impregnated magnetic Fe nanoparticles. This effective hydrogenation catalyst was attracted to an external magnet and so was easily separated from the reaction matrix. PMID:24605203 Duk Keun An of Kangwon National University has found (Chem. Lett. 2007, 36, 886.DOI: 10.1246/cl.2007.886)that by including NaOtBu, Dibal reduction of an ester such as 3 can be made to reliably stop at the aldehyde 4. By using the easily-prepared pentaflurophenyl ester 5, Panagiota Moutevelis-Minakakis of the University of Athens was able to reduce an acid to the alcohol 6. Lionel A. Saudan of Firmenich SA, Geneva has devised (Angew. Chem. Int. 6-Chloropyridazine-3-carbaldehyde Purity Price of 4-(Tert-butyl)pyridin-2-amine Ed. 2007, 46, 7473.DOI: 10.1002/anie.200701015)a Ru catalyst that will hydrogenate an ester such as 7 to the alcohol 8 without reducing an internal alkene.
Norio Sakai of the Tokyo University of Science has established (J. Org. Chem. 2007, 72, 5920.DOI: 10.1021/jo070814z)what promises to be a general route to ethers 10, by direct reduction of the corresponding ester 9. Hideo Nagashima of Kyushu University has developed (Chem. Commun. 2007, 4916.DOI: 10.1039/b711743d)a Ru catalyst that effected selective hydrogenation of an amide 11 to the amine 12 without reducing ketones or esters. Alternatively, Jason S. Tedrow of Amgen Inc., Thousand Oaks, CA has found (J. Org. Chem. 2007,72, 8870. DOI: 10.1021/jo701682c)that a protocol developed by Robert E. Maleczka, Jr. of Michigan State University was effective for reducing an aryl ketone 13 to the corresponding hydrocarbon 14 without reducing the amide.
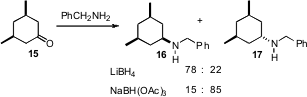
The stereocontrolledreductive amination of cyclic ketones such as 15 has been a continuing challenge. Shawn Cabral of Pfizer, Inc. in Groton, CT has reported (Tetrahedron Lett. 2007, 48, 7134.DOI: 10.1016/j.tetlet.2007.07.217)complementary reagent combinations, leading selectively to either 16 or17.
To control catalytic hydrogenation, it is often desirable to control the H2 supply. John S. McMurray of the University of Texas M. D. Anderson Cancer Center in Houston has shown (J. Org. Chem. 2007, 72, 6599.DOI: 10.1021/jo0706123)that Et3SiH is a convenient H2 source.
Nitro alkanes add to aldehydes to give nitro alkenes such as 20. Göran Hilmersson of Göteborg University has developed (Tetrahedron Lett. 2007, 48, 5707.DOI: 10.1016/j.tetlet.2007.05.105)conditions for the reduction of such a nitro alkene directly to the corresponding saturated amine 21. Erick M. Carreira of ETH Hönggerberg has coupled (Angew. Chem. Int. Ed. 2007, 46, 2078.DOI: 10.1002/anie.200604263)the enantioselective addition of benzylic nitro compounds such as 22 to aldehydes with catalytic hydrogenolysis, to deliver the secondary alcohol 25 in high ee.