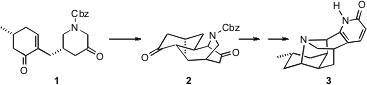
The pentacyclic alkaloid (+)-lyconadin A (3), isolated from the club moss Lycopodium complanatum, showed modest in vitro cytotoxicity. A key step in the first reported (J. PMID:23805407 Am. Chem. Soc. 2007, 129, 4148. DOI: 10.1021/ja070336+)total synthesis of 3, by Amos B. Smith III of the University of Pennsylvania, was the cyclization of 1 to 2.
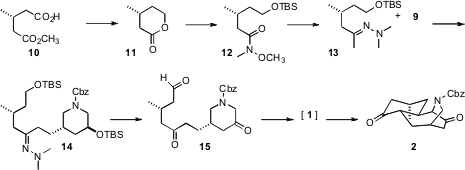
The pentacyclic skeleton of 3 was constructed around a central organizing piperidine ring 9. This was prepared from the known (and commercial) enantiomerically-pure lactone 4. 5-Bromopyridine-2-carbaldehyde web 5-Bromo-4-thiazolecarboxaldehyde web The akylated stereogenic center of 9 was assembled by diastereoselective hydroxy methylation of the acyl oxazolidinone 5 with s-trioxane, followed by protection. Reduction of the imide to the alcohol led to the mesylate 7, which on reduction of the azide spontaneously cyclized to give, after protection, the piperidine 8. Selectivedesilylation of the primary alcohol then enabled the preparation of 9.
The plan was to assemble the firstcarbocyclic ring of 3 by intramolecular aldol condensation of the keto aldehyde 15. The enantiomerically-pure secondary methyl substituent of 15 derived from the commercial monoester 10. Activation as the acid fluoride followed by selective reduction led to the volatile lactone 11. Opening of the lactone with H3CONHCH3. HCl gave, after protection, the Weinreb amide 12. Alkylation of the derived hydrazone 13, selectively on the methyl group, led, after deprotection, to 15. The intramolecular aldol condensation of 15 did deliver the unstable cyclohexenone 1. Under the acidic conditions of the aldol condensation, the enol derived from the piperidone added in a Michael sense, from the axial direction on the newly-formed ring, to give the trans-fused bicyclic diketone 2.
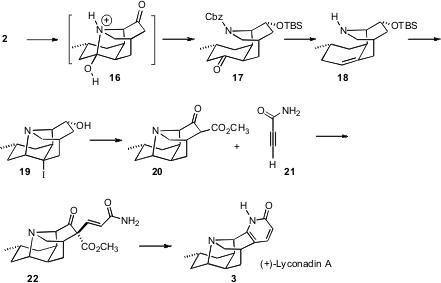
To move forward, it was necessary to epimerize 2 to the cis ring fusion, and also to differentiate the two ketones of 2. These two problems were solved simultaneously by deprotection and epimerization to the cis-fused hemiaminal 16.
Attempts to deoxygenate the tertiary alcohol of 16 failed, so instead, selective reduction followed by protection delivered 17. Reduction to the axial alcohol followed by dehydration with the Martin sulfurane then installed the trisubstituted alkene of 18. The C-N bond was re-established by exposure of 18 to NIS, leading to the crystalline 19. Activation of the derived ketone with Mander’s reagent followed by reductive deiodination (Et3SiH/Pd) gave 20, Michael addition of which with 21 led to 3.