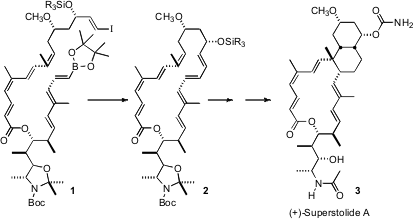
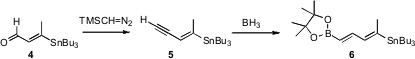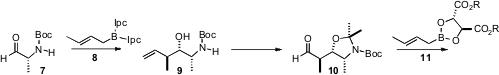(+)-Superstolide A (3), isolated from the New Caledonian sponge Neosiphonia superstes, shows interesting cytotoxicity against malignant cell lines at ~ 4 ng/mL concentration. The key transformation in the synthesis of 3 described (J. 212127-80-5 Formula Am. NH2-PEG8-OH web Chem. Soc. 2008, 130, 2722.DOI: 10.1021/ja710238h)by William R. Roush of Scripps Florida was the transannularDiels-Alder cyclization of 2, which established, in one step with high diastereocontrol, both the cis decalin and the macrolactone of 3. PMID:26895888
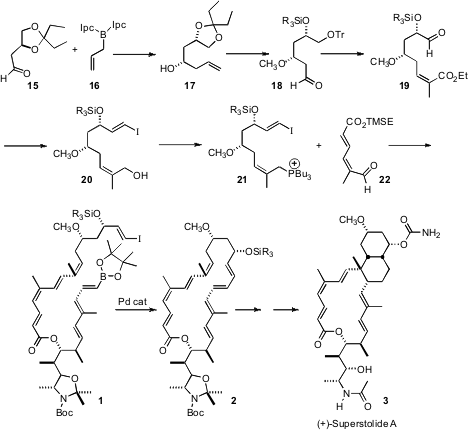
The octaene 1 was assembled from four stereodefined fragments. The first, the linchpin 6, was prepared from the stannyl aldehyde 4. Homologation gave the enyne 5, which on hydroboration and oxidation gave6.
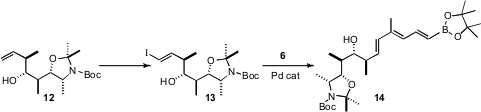
Earlier, Professor Roush had optimized the crotylation of the protected alaninal 7. In this case, the Brown reagent 8 delivered the desired Felkin product 9. Protection followed byozonolysis gave the aldehyde 10. Crotylation with the Roush-developed tartrate 11 then gave the alkene 12, setting the stage for conversion to the iodide 13. Coupling of 13 with 6 completed the preparation of 14.
The third component of (+)-superstolide A (3), the phosphonium salt 21, was assembled by Brown allylation of the aldehyde 15, to give 17. Protecting group interchange followed by ozonolysis delivered 18, which via Still-Gennari homologation was carried on to 21. Condensation with the fourth component, the aldehyde 22, and esterification with 14 then gave 1.
Under high dilution Suzuki conditions 1 was converted to 2. Storage in CDCl3 for five days, or brief warming, cyclized 2 to a single diastereomer of the transannular Diels-Alder product, that was carried on to (+)-superstolide A (3). While acyclic trienes comparable to 2 could be induced to cyclize, the transannular Diels-Alder reaction proceeded with much higher diastereocontrol.