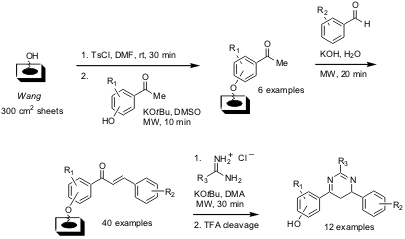
A recent publication by Helen E. Blackwell and co-workers from the University
of Wisconsin-Madison (Org. 1251013-26-9 Chemscene Lett. PMID:36717102 2004, 6, 2019.
DOI: 10.1021/ol049313f)
reports the multistep synthesis of a spatially addressed pyrimidine
library on planar membrane supports (SPOT synthesis). For all of the three steps
of the synthesis (see below) microwave irradiation was used to speed up the
chemistry on the planar support. Importantly, microwave irradiation did not
impact on the integrity of the cellulose support and the chemistry could easily
be scaled up employing other (non planar) types of cellulose supports.
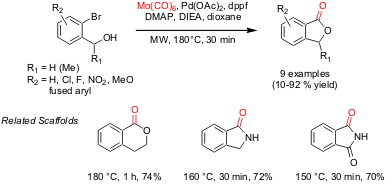
Tandem Carbonylation-Lactone Formation Reactions
Mathias Alterman and co-workers from Uppsala University have employed (Tetrahedron
Lett. Formula of Ruphos pd(crotyl)cl
2004, 45, 4635.
DOI: 10.1016/j.tetlet.2004.04.110) a tandem carbonylation-lactone formation reaction sequence for the
synthesis of phthalides. Optimum conditions involved the use of Mo(CO)6
as a solid source of CO, and Pd(OAc)2/dppf as a catalyst (5 mol%) at
180 °C. The microwave-assisted carbonylation-cyclization method was also applied
for the synthesis of other scaffolds, such as dihydroisocoumarins,
dihydroisoindones, and phthalimides.
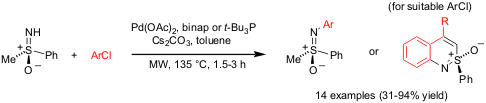
N-Arylation of Sulfoximines
Michael Harmata and coworkers from the University of Missouri-Columbia have
disclosed (Tetrahedron Lett. 2004, 45, 5233.
DOI: 10.1016/j.tetlet.2004.05.027) an efficient protocol for the Pd-catalyzed
N-arylation of enantiopure sulfoximines with aryl chlorides. Optimal
results were achieved by using Pd(OAc)2 as Pd-source and rac-binap
or t-Bu3P as ligands under microwave irradiation conditions.
For aryl chlorides bearing ortho-carbonyl substituents the corresponding
benzothiazines were obtained.
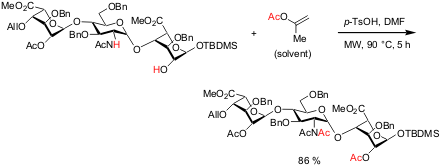
Heparin Oligosaccharide Synthesis
The synthesis of fully N-differentiated heparin oligosaccharides has
been demonstrated by Gregory J. S. Lohman and Peter H. Seeberger from the MIT (J.
Org. Chem. 2004, 69, 4081.
DOI: 10.1021/jo035732z).
One of the many synthetic steps involves the simultaneous installment
of an
N-diacetate and O-acetyl functionality in a trisaccharide building
block. Microwave irradiation in isopropenyl acetate as solvent in the presence
of
p-TsOH at 90 °C for 5 hours led to the desired product in 86 % yield.
This transformation could not be achieved under a variety of thermal conditions,
with only poor yields achieved even after several days.