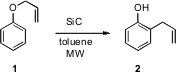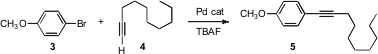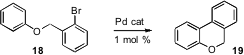There have been much discussion of the benefits of microwave irradiation. C. Oliver Kappe of the University of Graz, among others, has undertaken detailed studies directed toward rationalizing the many reports. In a recent contribution (J. Org. Chem. 2006, 71, 4651. 68634-02-6 site DOI: 10.1021/jo060692v), he has shown that by including sintered silicon carbide (SiC) cylinders as passive heating elements, one can use microwave irradiation to heat solvents such as toluene that do not directly absorb the radiation.
Sonogashira coupling is one of the most reliable of C-C bond forming reactions. Jin-Heng Li of Hunan Normal University, Changsha, has found (J. Org. Chem. 2006, 71, 379. DOI: 10.1021/jo051882t)that a Pd catalyst is effective for the coupling even in the absence of Cu or amines. Note that Pd-only coupling of an alkyne with an aryl halide was initially developed by Heck. Price of 1-Cyclobutylpiperazine The contribution of Sonogashira was to add Cu.
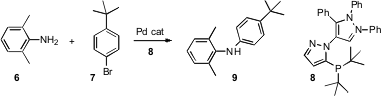
Although effective ligands for the Pd-catalyzed coupling of amines with aryl halides have been designed, many of these are patent protected. PMID:23600560 Robert A. Singer of Pfizer Global Research, Groton, CT has developed (Tetrahedron Lett. 2006, 47, 3727. DOI: 10.1016/j.tetlet.2006.03.132)a ligand 8 that is as effective as those previously reported, but that is not proprietary.
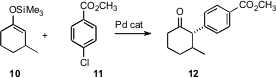
John K. Verkade of Iowa State University and John F. Hartwig, now at the University of Illinois, have reported (Angew. Chem. Int. Ed. 2006,45, 5852. DOI: 10.1002/anie.200601887)what appears to be a robust protocol for the Pd-catalyzed α-arylation of silyl enol ethers. If this procedure is as general as it appears to be, this will be a significant addition to the standard tools of organic synthesis. Professor Hartwig has also reported (J. Am. Chem. Soc. 2006, 128, 14800. DOI: 10.1021/ja064782t)conditions for coupling the Baldwin acyl anion equivalent to an aryl halide.
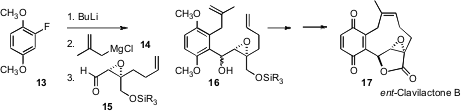
In the course of a synthesis of ent-clavilactone B (17), Anthony G. M. Barrett of Imperial College has described (J. Am. Chem. Soc. 2006, 128, 14042. DOI: 10.1021/ja0662671)an elegant three-component coupling. Addition of methallyl Grignard 14 to the benzyne derived from 13 delivered an intermediate aryl Grignard, that added to 15 to give 16.
In a feature article (Chem. Commun. 2006, 1253. DOI: 10.1039/b515481m)Keith Fagnou of the University of Ottawa has provided an overview of his elegant preparative and mechanistic work using intramolecular and intermolecular carbopalladation to arylate C-H positions on aromatic rings. The intramolecular reaction, which originally required 10 mol % of the Pd catalyst, can now be run with 1 mol % or less.
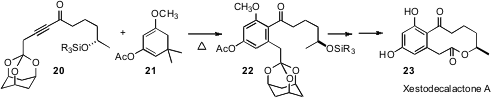
Rather than derivatize an existing benzene ring, it is sometimes more efficient to construct the ring. In the course of a synthesis of xestodecalactone A (23), Samuel J. Danishefsky of Columbia University showed (J. Am. Chem. Soc. 2006, 128, 14185. DOI: 10.1021/ja064270e)that the Diels-Alder addition of non-symmetrical allenyl and alkynyl dienophiles such as 20 to dienes such as 21 can proceed with high regiocontrol. Gerhard Hilt of the Philipps-Universität Marburg has described (Angew. Chem. Int. Ed. 2006, 45, 5204. DOI: 10.1002/anie.200601974)a complementary Diels-Alder approach to benzene construction that delivers meta substituted products.