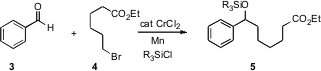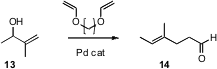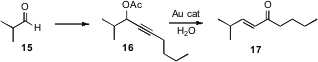Efficient new methods for the construction of C-C single, double and triple bonds have recently been reported. Erick M. Carreira of ETH Zurich has developed (Angew. Chem. 737790-46-4 site Int. PMID:24381199 Ed. 2007, 46, 4519.DOI: 10.1002/anie.200700575)a Co catalyst for the Markovnikov hydrocyanation of alkenes, as illustrated by the conversion of 1 to 2. This procedure was also found to be compatible with esters, amides, and ethers. A complementary catalyst to deliver the anti-Markovnikov product would certainly be welcome.
The reductive addition of an organic halide such as 4 to an aldehyde such as 3 is one of the basic transformations of organic synthesis. 1041026-70-3 structure Ludger A. Wessjohann of the Leibniz Institute of Plant Biochemistry, Halle, Germany, and Henri S. Schrekker of the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, have described (Tetrahedron Lett. 2007, 48, 4323.DOI: 10.1016/j.tetlet.2007.04.119)a convenient procedure that is catalytic in Cr, usingMn as the bulk reductant. This approach offers the additional advantage of producing the protected alcohol5 directly.
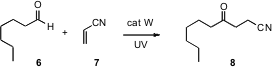
The direct generation of formyl radicals has been a long-sought goal. Maurizio Fagnoni of the Università degli Studi di Pavia has reported (Angew. Chem. Int. Ed. 2007, 46, 2531.DOI: 10.1002/anie.200604820)a promising approach, based on the photochemical activation of a tetrabutylammonium tungstate (TBADT) catalyst. Although the intermolecular additions required an activated alkene, intramolecular addition (not reported) to unactivated alkenes may work well.
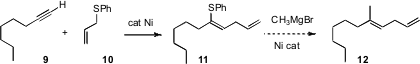
The stereocontrolled construction of alkenes is a continuing problem. Masato Tanaka of the Tokyo Institute of Technology has established (Org. Lett. 2007, 9, 263.DOI: 10.1021/ol062686r)a promising approach, based on the Ni-catalyzed addition of allyl phenyl sulfide10 to a terminal alkene 9 to give 11 as the dominant product. The authors do not report further transformations of 11, but it is likely that Ni-catalyzed coupling with, for instance, CH3MgBr, would convert 11 into 12.
Claisen rearrangement is also a good way to prepare disubstituted and trisubstituted alkenes. The simplest version of this reaction is two-carbon homologation by conversion of an allylic alcohol to the corresponding vinyl ether, followed by heating. Xudong Wei of Boehringer-Ingelheim, Ridgefield, CT has found (J. Org. Chem. 2007, 72, 4250.DOI: 10.1021/jo062548f)that commercial triethylene glycol divinyl ether served well as a high-boiling vinyl donor. Pd was used to catalyze the vinyl ether exchange.
An enone such as 17 would often be prepared by phosphonate homologation of the aldehyde 15. Liming Zhang of the University of Nevada, Reno has optimized (Adv. Synth. Catal. 2007, 349, 871. DOI: 10.1002/adsc.200600579)a practical alternative strategy, the key step of which was the Au-catalyzed rearrangement of the easily-prepared propargylic acetate 16.
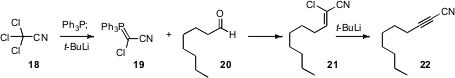
New methods for the construction of C-C triple bonds appear only rarely. Doo Ok Jang of Yonsei University has described (Tetrahedron Lett. 2007,48, 2299.DOI: 10.1016/j.tetlet.2007.01.157)such a method. A phosphorane 19 was prepared by combining the nitrile 18 with Ph3P and t-butyl lithium. Condensation of an aldehyde 20 with 19 gave the nitrile 21, which could be isolated. Alternatively, treatment with an additional equivalent of t-butyl lithium in the same pot converted 21 to the alkyne 22.