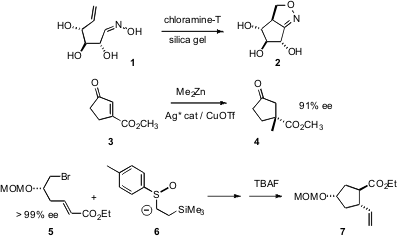
Several creative new approaches to carbocyclic ring construction have recently been reported. Tony K. PMID:23771862 M. Formula of (4-(Ethylsulfonyl)phenyl)methanamine Shing of the Chinese University of Hong Kong has found (Org. Lett. 2007, 9, 753.DOI: 10.1021/ol062873p)that intramolecular nitrile oxide cycloaddition (INOC) is compatible with multiple free OH groups, allowing direct conversion of carbohydrates to carbocycles. Amir H. Hoveyda of Boston College has overcome (Angew. Chem. Int. Ed. 2007,46, 1097.DOI: 10.1002/anie.200604511)the difficulty of enantioselectiveconjugate addition to substitutedcyclic enones by using an activating ester subsituent. These two approaches can be used to prepare both cyclopentanes andcyclohexanes. Charles K. Zercher of the University of New Hampshire, in the course of a synthesis of (+)-brefeldin A, has shown (J. 5-Chloro-4-methylpyridin-3-amine Order Org. Chem. 2007, 72, 4390.DOI: 10.1021/jo0701379)that highly functionalized cyclopentanes can also be prepared using the chiral sulfoxide conjugate addition developed by Toru.
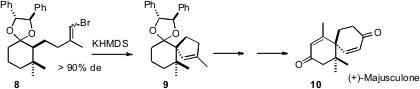
In a synthesis of the chamigrene sesquiterpene (+)-majusculone (10), we (J. Org. Chem. 2007, 72, 4098.DOI: 10.1021/jo070257g)separated the diastereomers of the ketal 8. On exposure to KHMDS, 8 cyclized to the enantiomerically-pure spiro alkene 9. The other diastereomer of 8 could be epimerized and recycled, so all the material could be brought forward to 9.
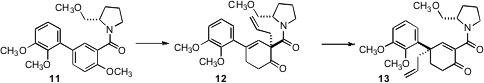
In a complementary enantioselective approach to cyclic alkylated quaternary centers, William P. Malachowski of Bryn Mawr College showed (J. Org. Chem. 2007, 72, 930.DOI: 10.1021/jo0621423)that reductive alkylation of the biphenyl amide 11 proceeded with high diastereocontrol, to give 12. On heating, 12 rearranged to 13, a key intermediate for the Malachowski synthesis of (-)-lycoramine (J. Org. Chem. 2007, 72, 6792).
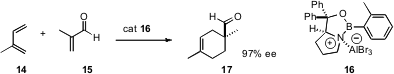
Diels-Alder cyclization is one of the most powerful tools for the construction of carbocyclic rings. It would be even more valuable if there were a general catalyst that worked well with less activated combinations of diene and dienophile. E. J. Corey of Harvard University has designed (J. Am. Chem. Soc. 2007, 129, 1498.DOI: 10.1021/ja068637r)the promising catalyst 16. Even with a relatively unreactive diene and dienophile combination such as 14 and 15, the catalyst 16 showed good reactivity and outstanding selectivity.
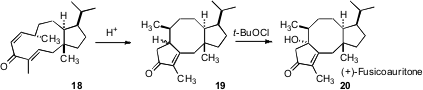
Enantio- and diastereoselective assembly of medium rings is particularly challenging. The biosynthesis of the tricyclic fusicoccanes, exemplified by (+)-fusicoauritone20, was hypothesized to proceed by acid-catalyzed cyclization of the dolabellane skeleton. David R. Williams of Indiana University has now shown (Angew. Chem. Int. Ed. 2007, 46, 915.DOI: 10.1002/anie.200603853)that exposure to acid of the diene 18, which has the dolebellane skeleton, gave clean Nazarov cyclization to the enone 19, which has the fusicoccane skeleton. Oxidation then completed the synthesis of (+)-fusicoauritone 20.