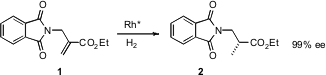
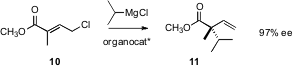A great deal of effort has gone into the enantioselective catalytic preparation of α-amino acids. Zhuo Zheng of the Dalian Institute of Chemical Physics has now (Org. Lett. 2006, 8, 3359. 3-Bromo-1,1-difluorocyclobutane Order DOI: 10.1021/ol0612399)developed an enantioselective catalytic route to particular class of β-amino acids.
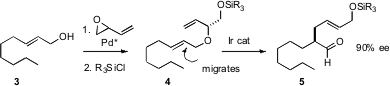
Barry M. Trost of Stanford University has described (Org. Lett. 6-Bromobenzo[d]isothiazole web 2006, 8, 6007. DOI: 10.1021/ol0624878)an elegant Claisen-based approach to alkylated aldehydes. Chiral Pd catalyzed coupling of 3 with racemic butadiene monoepoxide followed by protection gave 4 in 91% ee, as the Trost group had previously reported. Selective migration of the more substituted alkene followed by Claisen rearrangement then gave the aldehyde 5.
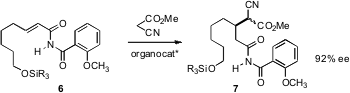
Yoshiji Takemoto of Kyoto University has devised (J. Am. Chem. Soc. PMID:24187611 2006, 128, 9413. DOI: 10.1021/ja061364f)a thiourea-based organocatalyst that mediates enantioselectiveMichael addition, using tuned acceptors such as 6.
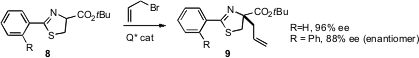
Hyeung-geun Park and Sang-sup Jew of Seoul National University have found (J. Org. Chem. 2006, 71, 8276. DOI: 10.1021/jo061107t)that chiral phase transfer catalysts can be used to direct alkylation of 8 (R=H) with high ee. Even more remarkably, alkylation of 8 (R=phenyl) proceeds to give the enantiomer of 9 in almost as high ee.
The direct preparation of tetraalkylated quaternary centers with control of absolute configuration has been a challenge. Several new solutions to this important problem are highlighted here. Amir H. Hoveyda of Boston College has devised (J. Am. Chem. Soc. 2006, 128, 15604. DOI: 10.1021/ja067456m)a chiral organocatalyst that directs the SN2′ coupling of Grignard reagents with chlorides such as 10, to give 11 in high ee.
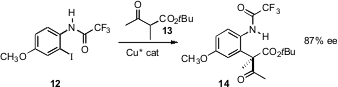
Dawei Ma of the Shanghai Institute of Organic Chemistry has found (J. Am. Chem. Soc. 2006, 128, 16050. DOI: 10.1021/ja066991j)that an aryl iodide such as 12 bearing an o-amido group is reactive enough to undergoUllman coupling with a β-keto ester such as 13. Using a chiral Cu catalyst, the product 14 was formed in high ee.
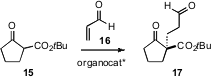
Li Deng of Brandeis University, extending his studies of cinchona-derived catalysts, has found (Angew. Chem. Int. Ed. 2006, 45, 4301. DOI: 10.1002/anie.200600867)that they mediate Michael addition of donors such as 15 to unsaturated aldehydes such as acrolein16. This appears to be a general method for the construction of cyclic quaternary centers with high ee.
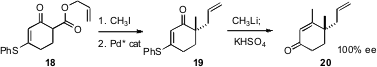
Professor Trost has also reported (Angew. Chem. Int. Ed. 2006, 45, 3109. DOI: 10.1002/anie.200504421)what appears to be a general strategy for the assembly of cyclic alkylated quaternary centers with high ee. The easily prepared β-keto ester 18 can be alkylated efficiently. Exposure of the alkylated product to a chiral Pd catalyst established, via loss of CO2, the cyclic quaternary center in high ee. The ketone 19 so produced is versatile, easily converted into a variety of other cyclohexane derivatives.