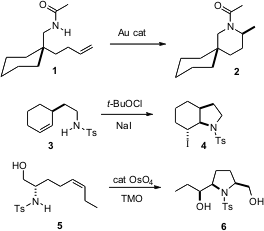
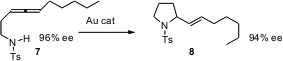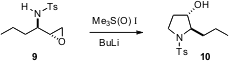There have been significant advances in the intramolecular addition of amines to alkenes. Ross A. BuyPyrazine-2,6-dicarboxylic acid Widenhoefer of Duke University has found (Chem. Commun. Formula of 2-Bromo-N,N-diphenylaniline 2006, 4143. DOI: 10.1039/b608638a)conditions for the long-sought intramolecular hydroamination of a carboxamide 1 to the cyclic amine 2. Satoshi Minakata and Mitsuo Komatsu of Osaka University have shown (Org. Lett. 2006, 8, 3335. DOI: 10.1021/ol061182q)that cyclization of alkenyl sulfonamides with in situ generated t-butylhypoiodite proceeds with high diastereocontrol. Timothy J. Donohoe of the University of Oxford has coupled (Angew. PMID:23996047 Chem. Int. Ed. 2006, 45, 8025. DOI: 10.1002/anie.200603240)osmylation with intramolecular amination, allowing the cyclization of 5 to 6 with total diasterocontrol.
Allenes such as 7 are readily prepared in high enantiomeric excess. Yoshinori Yamamoto of Tohoku University has established (Tetrahedron Lett. 2006, 47, 4749. DOI: 10.1016/j.tetlet.2006.04.087)that the Au-catalyzed cyclization of 7 leads to 8 with nearly perfect enantiocontrol.
Sharpless aziridination followed by aza-Payne rearrangement allows easy access to epoxy sulfonamides such as 9. David Hodgson of the University of Oxford has found (Chem. Commun. 2006, 3226. DOI: 10.1039/b606583j)that on exposure to dimethylsulfoxonium methylide, 9 is converted to the hydroxypyrrolidine 10. The reaction is presumably proceeding by epoxide opening followed by displacement of the sulfoxonium leaving group by the sulfonamide anion.
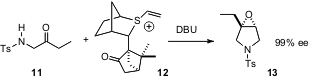
In a conceptually related development, Varinder K. Aggarwal of the University of Bristol has designed (Angew. Chem. Int. Ed. 2006, 45, 7066. DOI: 10.1002/anie.200602782)the vinyl sulfonium salt 12, condensation of which with an amino ketone 11 proceeds to give the epoxypyrollidine 13 in high enantiomeric excess.
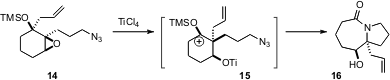
Azides can be seen as chemical chameleons, serving sequentially both as nucleophilic and then as electrophilic centers. This is nicely illustrated by the observation (Org. Lett. 2006, 8, 5271. DOI: 10.1021/ol062116r)by Yong Qiang Tu of Lanzhou University that the cation 15 from pinacol rearrangement of 14 is trapped by the pendant azide to give, after loss of N2, the lactam 16.
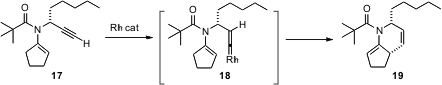
Chulbom Lee of Princeton University has applied (J. Am.Chem. Soc. 2006, 128, 6336. DOI: 10.1021/ja0619758)modern understanding of mechanistic organometallic chemisty to the preparation of cyclic amines. Terminal alkynes such as 17 are rearranged by Rh catalysts to the corresponding vinylidene complex 18. This is electophilic enough to react with the tethered enamine, leading with high diasterecontrol to the amine 19.