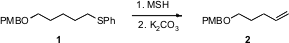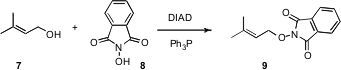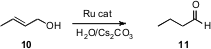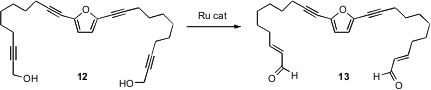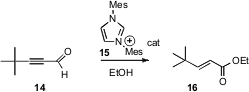The thermal elimination of aryl sulfoxides is widely used, but requires elevated temperatures. Jun-ichi Matsuo and Hiroyuki Ishibashi of Kanazawa University have now shown (Org. Lett. Price of 168892-66-8 2006, 8, 6095.DOI: 10.1021/ol062620w)that exposure of a sulfide 1 to O-mesitylenesulfonylhydroxylamine (MSH) at room temperature followed by K2CO3 led to smoooth elimination to the alkene2.
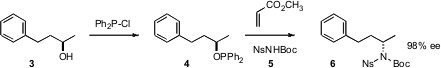
Teruaki Mukaiyama of Kitasato Institute in Tokyo recently reported (Chem. Lett. Formula of 674799-96-3 2006, 35, 456.DOI: 10.1246/cl.2006.456)a convenient protocol for Mitsunobu coupling. The alcohol to be displaced is first converted into the diphenylphospinite 4. PMID:25804060 Exposure to methyl acrylate and the nucleophile 5 then leads to the coupled product6 with near-perfect inversion of absolute configuration.
Another improvement in Mitsunobu coupling comes (Tetrahedron Lett. 2006, 47, 5151. DOI: 10.1016/j.tetlet.2006.05.070)from David W. Knight at Cardiff University. He has found that after carrying out a Mitsunobu reaction in the usual way, with Ph3P and diethylazodicarboxylate (DEAD) or diisopropylazodicarboxylate (DIAD), filtration through a plug of silica gel with CH2Cl2, exposure to 15% aqueous H2O2, and again filtration through a plug of silica gel with CH2Cl2 led to pure product9 as a powdery solid. This protocol is easily scaled up.
The Ru-catalyzed "redox" isomerization of an allylic alcohol 10 to the corresponding aldehyde 11 is a useful synthetic transformation. Victorio Cadierno, José Gimeno and José A. Sordo of the Universidad de Oviedo have described (J. Am. Chem. Soc. 2006, 128, 1360. DOI: 10.1021/ja054827a)a detailed study of this reaction. Secondary alcohols also participate efficiently, leading to ketones.
Barry M. Trost of Stanford University has shown (Org. Lett. 2006, 8, 4461.DOI: 10.1021/ol0615836)that propargyl alcohols such as 12 can also be isomerized. The product is the α,β-unsaturated aldehyde 13.
Kirsten Zeitler of the Universität Regensburg has found (Org. Lett. 2006, 8, 637. DOI: 10.1021/ol052826h)that acetylenic aldehydes such as 14 can also be isomerized, in this case with the organocatalyst 15. The product is the ester 16.
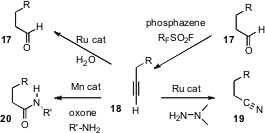
A remarkable series of transformations around terminal alkynes have recently been reported. Ilya M. Lyapkalo of the Institute of Chemical & Engineering Sciences, Singapore, has shown (Angew. Chem. Int. Ed. 2006, 45, 4019.DOI: 10.1002/anie.200504594)that aldehydes 17 react with the inexpensive nonafluorobutanesulfonyl fluoride in the presence of a phosphazene base to give first the enol sulfonate, and then the terminal alkyne 18. Lukas Hintermann of RWTH Aachen University has developed (Org. Lett. 2006, 8, 5853. DOI: 10.1021/ol062455k)a Ru catalyst for the inverse transformation, the hydration of the terminal alkyne 18 to the aldehyde 17. Several years ago, Yoshiya Fukumoto of Osaka University reported (Organomet. 2002, 21, 3845. DOI: 10.1021/om020588d)the Ru-catalyzed conversion of terminal alkynes to the corresponding nitriles 19. This has been little used, but we have found that it works well. The oxidation of alkynes 18 to the amide 20 described (J. Am. Chem. Soc. 2006, 128, 14796. DOI: 10.1021/ja064479s)by Man-Kin Wong and Chi-Meng Che of the University of Hong Kong also works well with internal alkynes.