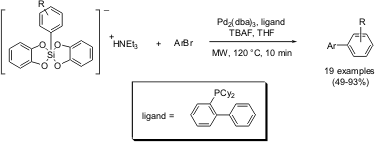
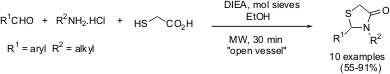Somewhat related to the well-knownSuzuki cross-coupling reaction involving organoboron reagents, is the so-calledHiyama coupling of organosilanes with halides and triflates to form unsymmetrical biaryl compounds. Seganish and DeShong from the University of Maryland have described rapid, microwave-promoted Hiyama couplings of bis(catechol) silicates with aryl bromides (Org. Lett. 169566-81-8 manufacturer 2004, 6, 4379.DOI: 10.1021/ol048044q). Suitable reaction conditions entailed the use of 5 mol% of Pd2(dba)3 as a palladium source, 5 mol% of 2-(dicyclohexylphosphanyl)biphenyl as ligand, and 1.5 equivalents of tetrabutylammonium fluoride (TBAF) in tetrahydrofuran as solvent. Exposure of the reaction mixture to microwave irradiation at 120°C for 10 minutes typically provided good yields of biaryl coupling products for a wide range of substrates. The only functional group that was found to fail in the coupling studies was the amino group, probably as a result of catalyst poisoning. Price of 457613-78-4
Sonogashira/Heteroannulation Strategies
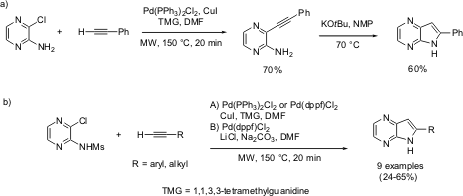
Hopkins and Collar have reported a one-potSonogashira/heteroannulation strategy for the synthesis of 6-substituted-5H-pyrrolo[2,3-b]pyrazines (Tetrahedron Lett. PMID:24605203 2004, 45, 8631.DOI: 10.1016/j.tetlet.2004.09.152). The reaction could either be performed in a two-step protocol, by first performing a classical Sonogashira coupling on 2-amino-3-chloropyrazine, followed by base-induced cyclization (reaction a), or in a one-step method by reacting the corresponding sulfonamide,N-(3-chloropyrazin-2-yl)-methanesulfonamide, directly with terminal acetylenes in the presence of suitable palladium catalyst (3 mol%) (reaction b). The microwave conditions (150 °C, 20 minutes) tolerated much functional diversity (both electron-withdrawing and electron-donating substituents). Halogens as well as cyano groups were also tolerated, along with silyl protecting groups.
β-Glycosylamine Synthesis
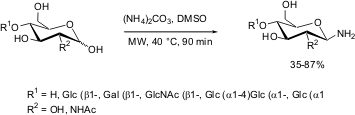
Bajugam and Flitsch have described (Org. Lett. 2004, 6, 4001.DOI: 10.1021/ol048342n). the synthesis of glycosylamines from mono-, di-, and trisaccharides via direct microwave-assisted Kochetkov amination. The reaction was found to be effective with only 5-fold excess (w/w) of ammonium carbonate over sugar compared to 40-50-fold excess needed under thermal conditions. All transformations were completed within 90 minutes in dimethylsulfoxide as solvent while maintaining the vessel temperature at an apparent 40°C, using the "heating-while-cooling" technique.
4-Thiazolidinones via Multicomponent Chemistry
Miller and co-workers reported a one-pot protocol for the preparation of thiazolidin-4-ones by condensation of aromatic aldehydes, amines and mercaptoacetic acid in ethanol (Synlett2004, 2357.DOI: 10.1055/s-2004-832811). The optimized procedure involved microwave irradiation of a mixture of amine hydrochloride, aldehyde and mercaptoacetic acid (molar ratio 1:2:3) in the presence of 1.25 equivalents ofN,N-diisopropylethylamine (DIEA) base in ethanol at 120 °C for 30 minutes at atmospheric pressure.
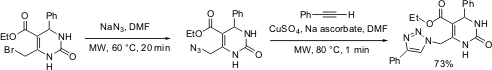
Cu(I)-Catalyzed Azide-Acetylene Ligations ("Click Chemistry")
We have exploited a microwave-assisted version of the copper(I)-catalyzed azide-acetylene ligation process ("click chemistry") for the preparation of 6-(1,2,3-triazol-1-yl)-dihydropyrimidones (J. Comb. Chem. 2004, 6, 884.DOI: 10.1021/cc0498938). Here a suitable heterocyclic azide intermediate (obtained by microwave-assisted azidation) was treated with phenyl acetylene inN,N-dimethylformamide employing 2 mol% copper(II) sulfate/sodium ascorbate as catalyst precursor. After completion of the cycloaddition process the triazole product can be precipitated in high yield (73%) and purity by addition to ice/water. For the model reaction displayed in the Scheme below full conversion at room temperature required one hour. By carrying out the same reaction utilizing controlled microwave heating at 80 °C, complete conversion was achieved within 1 minute. A library of twenty seven 6-(1,2,3-triazol-1-yl)-dihydropyrimidones was prepared with 4 points of diversity.
For certain substrates, Fokin, Van der Eycken and co-workers discovered that the azidation and ligation step can be carried out in a one-pot fashion, thereby simplifying the overall protocol (Org. Lett. 2004, 7, 4223.DOI: 10.1021/ol048341v). This procedure eliminates the need to handle organic azides, as they are generated in situ.
Recent Reviews:
Kappe, C. O. "Controlled Microwave Heating in Modern Organic Synthesis", Angew. Chem. Int. Ed. 2004, 43, 6650.DOI: 10.1002/anie.200400655 (> 400 references).