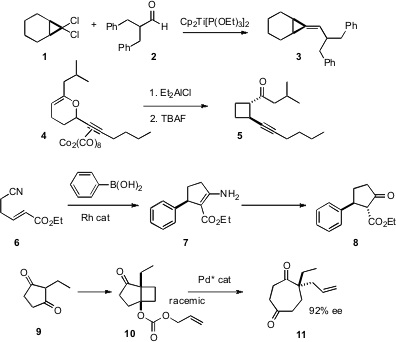
Several creative new approaches to carbocyclic ring construction using transition metals have recently been reported. Dichlorocyclopropanes such as 1 are easily prepared by the addition of dichlorocarbene to the corresponding alkene. Takeshi Takeda of the Tokyo University of Agriculture and Technology has shown (Tetrahedron Lett. Price of 109781-47-7 2007, 48, 3521. DOI: 10.1016/j.tetlet.2007.03.110)that the Ti-mediated coupling of 1 with an aldehyde or ketone proceeded to give the alkylidene cyclopropane 3. Joseph P. PMID:23381601 A. Harrity of the University of Sheffield has found (J. Org. Chem. Geranylgeraniol custom synthesis 2007, 72, 3467. DOI: 10.1021/jo0701132)that the Co-supported Ferrier rearrangement he has developed can be extended to dihydropyrans such as 4, to give the cyclobutane 5 with high diastereocontrol. Rh-catalyzed conjugate addition of boronic acids gives an intermediate enolate that will react with a variety of tethered electrophiles including, as Masahiro Murakami of Kyoto University has demonstrated (Org. Lett. 2007, 9, 741. DOI: 10.1021/ol062882y), a tethered nitrile. This reaction worked well to form 5- and 6-membered rings. 2-Ethyl cyclopentane 9 is prochiral. Siegfried Blechert of the Technische Universität Berlin has devised (Angew. Chem. Int. Ed. 2007, 46, 3966. DOI: 10.1002/anie.200604553)an enantiomerically-pure Pd catalyst that converted racemic 10, derived from 9, into the cycloheptanone 11 with high ee.
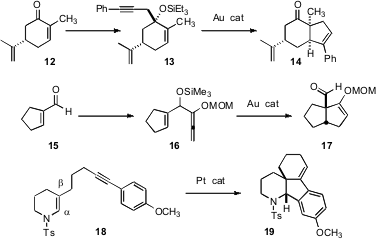
Transition metals have also been used to construct multiple ring systems. Stefan F. Kirsch of the Technisches Universität München has found (Angew. Chem. Int. Ed. 2007, 46, 2310. DOI: 10.1002/anie.200604544)an Au catalyst that will rearrange an alkoxy enyne such as 13 to the ketone 14. This is a new method for annealing a new ring onto a cyclic enone. Liming Zhang of the University of Nevada, Reno has described (J. Am. Chem. Soc. 2007, 129, 6398. DOI: 10.1021/ja0717717)a complementary process, the Au-catalyzed conversion of 16 to 17.
Enamides such as 18 are nucleophilic at the β carbon, leading to an electrophilic center at the α carbon. Gregory R. Dake of the University of British Columbia has used (Org. Lett. 2007, 9, 367.DOI: 10.1021/ol062939g)a Pt catalyst to bring out this inherent reactivity, allowing the conversion of 18 to the tricyclic19.
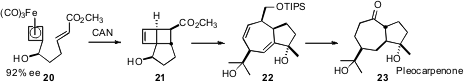
Marc L. Snapper of Boston College has been exploring synthesis applications of Fe-cyclobutadiene complexes. In a recent contribution (J. Am. Chem. Soc. 2007, 129, 486. DOI: 10.1021/ja0674340)he has shown that oxidative decomplexation of the enantiomerically-enriched complex 20 delivered the tricyclic adduct 21 with good diastereocontrol. Cyclopropanation followed by thermal fragmentation led to the 7-5 system 22, which was carried on to the guaiane sesquiterpene pleocarpenone23.