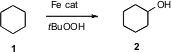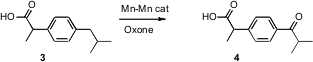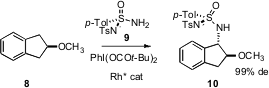Direct conversion of an unactivated C-H bond to a C-O, C-N or C-C bond is a powerful way to add valuable complexity to a substrate. While this is by no means a new approach (Friedel-Crafts acylation converts a C-H bond to a C-C bond), there have been several useful new developments.
Biosynthetic C-H oxygenation is mediated by the several isozymes of Fe-centered cytochrome P-450. Debkumar Bandyopadhyay of the Indian Institute of Technology, New Delhi has developed (Chem. Comm. 2006, 4823.DOI: 10.1039/b611988c)an Fe complex that catalyzed the oxidation of cyclohexane 1 to cyclohexanol 2, with about 20 turnovers. PMID:23671446 Shinobu Itoh of Osaka City University has devised (Chem. Comm. Tributyl(1-ethoxyethenyl)stannane Purity 2006, 4016.DOI: 10.1039/b608311k)a Ni catalyst that effected the same conversion with about 600 turnovers, usingMCPBA as the bulk oxidant.
More complex substrates are also interesting. Robert H. 6-Chloro-5H-benzo[a]phenoxazin-5-one In stock Crabtree and Gary W. Brudvig of Yale University have shown (Science 2006, 312, 1941. DOI: 10.1126/science.1127899)that a Mn-Mn complex catalyzed the oxidation of 3 to 4 with high selectivity.
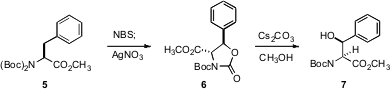
David Crich, now at Wayne State University, has reported (J. Org. Chem. 2006, 71, 7106.DOI: 10.1021/jo061159i)a different approach to C-H functionalization. Exposure of phenylalanine derivatives such as 5 to NBS gave the bromide, presumably as a epimeric mixture. Solvolysis lead to the product 6 and thus to 7 as single diastereomers.
C-H bonds can also be converted to C-N bonds. Paul Müller of the University of Geneva and Robert H. Dodd and Philippe Dauban of Gif-sur-Yvette have reported (Angew. Chem. Int. Ed. 2006, 45, 4641.DOI: 10.1002/anie.200601248)that oxidation of 9 in the presence of the prochiral 8 led to 10 in high de. David A. Powell of Merck Frosst Canada has also described (Org. Lett. 2006, 8, 6031.DOI: 10.1021/ol062514u)a protocol for the direct amidation of allylic and benzylic C-H bonds.
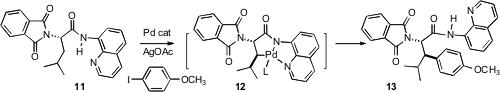
One of the most powerful of C-H functionalizations is the conversion to a C-C bond. E. J. Corey of Harvard University found (Org. Lett. 2006, 8, 3391. DOI: 10.1021/ol061389j)that oxidation of the amino acid derivative 11 with Pd salts led to the C-H activated product 12. If the palladation was run in the presence of an aryl iodide, intermediate 12 coupled to give 13, with high diastereocontrol.
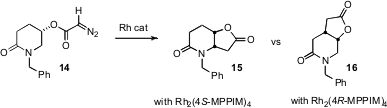
Rh-mediated carbene transfer is also a powerful method for converting a C-H to a C-C bond. Andrew G. H. Wee of the University of Regina has described (Chem. Commun. 2006, 3732.DOI: 10.1039/b606436a)the cyclization of the enantiomerically-pure diazo acetate 14. The C-H bond adjacent to the N is the more reactive, and is the site of insertion with racemic catalysts, leading to 15. With the enantiomerically-pure catalyst Rh2(4S-MPPIM)4, 15 was the only product observed. With the enantiomeric catalyst Rh2(4R-MPPIM)4, the chirality of the catalyst dominated, so the major product was 16. The Rh2(4R-MPPIM)4, and Rh2(4S-MPPIM)4 catalysts were developed by Michael P. Doyle of the University of Maryland.