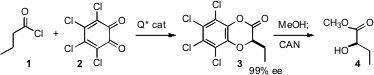
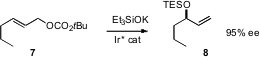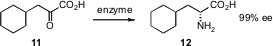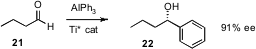Thomas Lectka of Johns Hopkins University has reported (J. PMID:24487575 Am. Chem. Soc. 2006, 128, 1810. DOI: 10.1021/ja058077g)an elegant method for the enantioselectiveα-hydroxylation of carboxylic acids. Chiral amine catalyzed condensation of the derived ketene with chloranil 2 proceeded with high enantioselectivity, to give the lactone 3, which was converted to 4 by transesterification and oxidative deprotection.
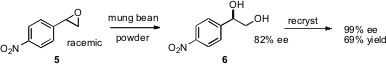
The power of enzymatic transformation is illustrated by the work (Org. Lett. 5-Bromo-2-chloropyridin-4-ol custom synthesis 2006, 8, 1737. DOI: 10.1021/ol060407u)of Jian-He Xu of the East China University of Science and Technology in Shanghai. 1956434-67-5 supplier Both enantiomers of styrene oxides such as 5 are converted by mung bean powder largely to the same enantiomer of the product diol6. It would be interesting to try the mung bean powder with (meso) cis stilbene oxide.
One of the simplest routes to an enantiomerically-pure secondary alcohol is the enantioselective displacement of a primary allylic leaving group. Erick M. Carreira of the ETH Zürich has extended (Angew. Chem. Int. Ed. 2006, 45, 6204. DOI: 10.1002/anie.200602408)this approach, using the potassium salt of triethylsilanol as the nucleophile and a chiral Ir catalyst.
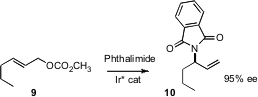
This same approach has been used to prepare enantiomerically-pure secondary amines, mainly using benzylamines as the nucleophile. Günter Helmchen of the Universität Heidelberg has extended (Angew. Chem. Int. Ed. 2006, 45, 5546. DOI: 10.1002/anie.200601472)this approach, using amine nucleophiles bearing more readily removable protecting groups.
L-amino acids are available from natural sources. D-amino acids have been much more expensive. Scott J. Novick of BioCatalytics, Inc., Pasadena, CA, through a combination of rational and random mutagenesis, has developed (J. Am. Chem. Soc. 2006, 128, 10923.DOI: 10.1021/ja0603960)a D-amino acid dehydrogenase. This new enzyme converts α-keto acids such as 11 into the corresponding D-amino acids in high ee.
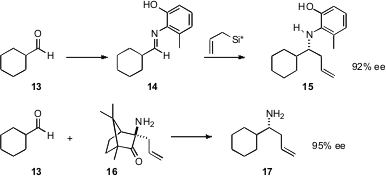
Asymmetric allylation of aldehydes, to prepare enantiomerically pure homoallylic alcohols, has become a reliable tool for organic synthesis. James L. Leighton of Columbia University (Org. Lett. 2006, 8, 6119.DOI: 10.1021/ol062589y)and Shu Kobayashi of the University of Tokyo (J. Am. Chem. Soc. 2006, 128, 11038.DOI: 10.1021/ja064106r)have each developed chiral reagents to add allyl to an imine, to give the enantiomerically pure homoallylic secondary amine.
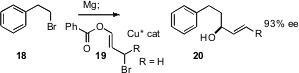
Ben L. Feringa of the University of Groningen has assembled (J. Am. Chem. Soc. 2006, 128, 15572.DOI: 10.1021/ja065780b)a powerful strategy for the three-carbon homologation of an alkyl halide, based on the chiral catalyst mediated coupling of the Grignard reagent derived from the halide with the bromoester 19 (R=H). A key question is whether this coupling works equally well with secondary bromides (R=alkyl).
The enantioselective addition of alkyl organometallics to aldehydes has been available for several years. Han-Mou Gau of National Chung-Hsing University in Taichung has now introduced (J. Am. Chem. Soc. 2006, 128, 14808.DOI: 10.1021/ja062080y)a protocol for the chiral catalyst mediated enantioselective addition of aryl organometallics to aldehydes.