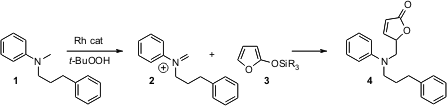
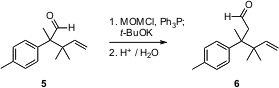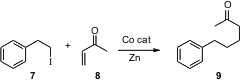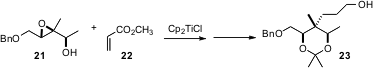Most methods for C-C bond formation involve the coupling of two already-functionalized carbons. Michael P. Doyle of the University of Maryland has developed (J. Am. Chem. Soc. (S)-2-Azido-3,3-dimethylbutanoic acid web 2006, 128, 5648. DOI: 10.1021/ja061146m)an elegant method for directly homologating C-H bonds adjacent to amines. Formula of 1H-Indole-6-carbaldehyde In the presence of aqueoust-butylhydroperoxide, amines such as 1 were oxidized to the corresponding iminium ions (e.g. 2), which coupled with the siloxy furan 3 to give4.
The one-carbon homologation of an aldehyde to the extended aldehyde has usually been carried out with Ph3P=CH-OCH3. Mukund G. Kulkarni of the University of Pune has described (Tetrahedron Lett. 2006, 47, 3027. PMID:25040798 DOI: 10.1016/j.tetlet.2006.03.012)an inexpensive alternative. Combination of commercial MOM-Cl with Ph3P in THF followed by the addition of t-BuOK gave a reagent that converted 5 to the homologated enol ether, hydrolysis of which gave 6.
Conjugate addition of an alkyl halide to an enone usually entails initial formation of the Grignard reagent. Paul Knochel has championed the Cu-mediated conjugate addition of alkyl zinc halides. Chien-Hong Cheng of the National Tsing Hua University, Hsinchu, has found (J. Org. Chem. 2006, 71, 655. DOI: 10.1021/jo052065w)that the reagent prepared by in situ reduction of an alkyl halide with Zn powder in the presence of a Co catalyst adds in a conjugate fashion to unsaturated ketones, esters, nitriles and sulfones. Like the Knochel protocol, this procedure is compatible with an ester on the alkyl halide.
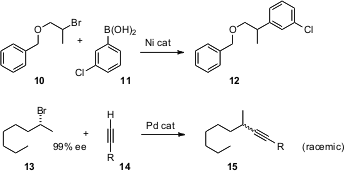
Coupling reactions that have long been known to work well for halides attached to sp2-hybridized carbons are now being extended to halides attached to sp3-hybridized carbons. Gregory C. Fu of MIT has worked out (J. Am. Chem. Soc. 2006, 128, 5360. DOI: 10.1021/ja0613761)conditions for Ni-catalyzed Suzuki coupling to secondary halides (10 + 11 → 12), and Frank Glorius of the Philipps-Universität, Marburg has extended (Tetrahedron Lett. 2006, 47, 2925. DOI: 10.1016/j.tetlet.2006.02.111)Sonogashira coupling to primary and secondary halides (13 + 14 → 15). This latter method is compatible with esters, epoxides and alkenes. Although bond formation is efficient, the enantiomeric excess of the leaving group is not maintained.
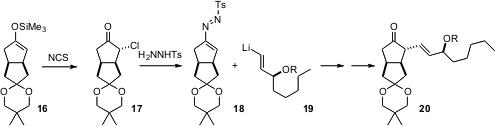
The α-alkenylation of a ketone is a delicate process, as the products (e.g. 20) easily go on to (undesired) conjugation. In the course of a synthesis of carbacyclin (J. Org. Chem. 2006, 71, 4642. DOI: 10.1021/jo060551t), Hans-Joachim Gais of RWTH Aachen optimized a protocol based on halogenation of a defined silyl enol ether 16.
The enantioselective construction of quaternary alkylated centers is a continuing challenge. Tushar Kanti Chakraborty of the Indian Institute of Chemical Technology, Hyderabad has shown (J. Org. Chem. 2006, 71, 3321. DOI: 10.1021/jo0600961)that addition to methyl acrylate 22 of the radical from Ti(III) reduction of the Sharpless-derived epoxy alcohol 21 proceeds with high facial selectivity, leading to the acetonide 23.