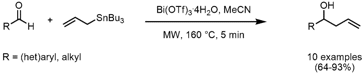
Thierry Ollevier and Zhiya Li from Université Laval, Quebec, have reported on the bismuth-catalyzedSakurai reaction toward the synthesis of homoallylic alcohols (Eur. J. 1505818-73-4 web Org. Chem. 1246761-84-1 supplier 2007, 34, 5665.DOI: 10.1002/ejoc.200700817). Several aldehydes were successfully converted to the corresponding homoallylic alcohols by reaction with 1 equiv of allyltributylstannane and only 0.5 mol% of bismuth(III)-triflate (Bi(OTf)3•4H2O) as catalyst. By microwave irradiation at 160 °C for 5 min, the alcohols were obtained in good to excellent yields without any by-product formation..
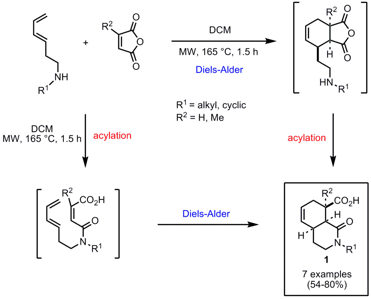
Tandem Diels-Alder/Acylation Sequence
A one-pot Diels-Alder/acylation (or vice versa) sequence for the synthesis of isoquinol-1-one-8-carboxylic acids 1 has been developed by the group of Jeffrey Aubé from the University of Kansas (J. PMID:25804060 Comb. Chem. 2007, 9, 1188. DOI: 10.1021/cc700127f). A set of six dienes containing an attached secondary amine functionality were reacted with maleic anhydride (R2 = H) or citraconic anhydride (R2 = Me) at 165 °C for 1.5 h to afford the isoquinolone products 1 in one step. In addition, the synthesis of the aminodiene precursors was performed under microwave conditions as well by displacement of a mesylate with the corresponding primary amine at 130 °C within 1 h in MeCN. The six carboxylic acid scaffolds obtained by the reaction with maleic anhydride were further reacted with 12 diverse amines to generate a 72-member isoquinolone-amide library. However, this step was performed at room temperature.
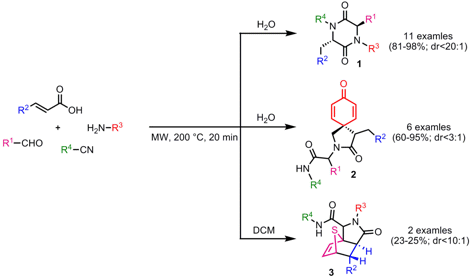
Generation of Molecular Diversity from a Multicomponent Reaction
Peter Andreana and Soumava Santra from Wayne State University, Michigan, have investigated that small molecule diversity can be obtained in a single-step starting from standard Ugi multicomponent reaction building blocks (Org. Lett. 2007, 9, ASAP.DOI: 10.1021/ol702256t). Depending on the solvent and sterics of the substituents, in particular of the amine and isocyanide precursors, three different scaffolds can be generated. When water is employed as protic solvent either 2,5-diketopiperazines 1 via an aza-Michael reaction or 2-azaspiro[4.5]deca-6,9-diene-3,8-diones 2 from a 5-exo Michael cyclization are obtained due to stabilization of zwitterionic intermediates by the protic solvent. When the reactions are performed in aprotic DCM only the acyclic Ugi products, which are formed in the first reaction step prior to intramolecular cyclizations, could be isolated. If bulky R4-substituents (t-Bu, i-Pr, cyclic alkyl) are present either product 2 or the acyclic Ugi product are accessible, depending on the amine substitution pattern. The tricyclic lactam product 3 was obtained via an intramolecular thiophene-derivedDiels-Alder reaction when thiophene-2-carboxaldehyde in DCM was used. Interestingly, direct conversion to products 1-3 could only be achieved by microwave irradiation and not with conventional heating.
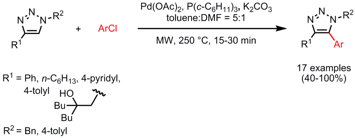
Direct Arylation of Substituted Triazoles
The groups of Hideki Yorimitsu and Koichiro Oshima from Kyoto University have disclosed palladium-catalyzed direct arylations of 1,4-disubstituted triazoles with aryl chlorides (Chem. Asian. J. 2007, 2, 1430.DOI: 10.1002/asia.200700206). Slightly longer reaction times (20-30 min) were required in order to achieve high product yields when hexyl-substituted triazoles (R1 = n-C6H13) are employed as precursors. Interestingly, when tricyclohexylphosphine [P(c-C6H11)3] is used as ligand, aryl chlorides were superior compared to aryl bromides and iodides. A solvent mixture of toluene and DMF (5:1) is crucial for the outcome of the reaction, since pure toluene can not be heated to 250 °C whereas pure DMF afforded the products in lower yields. It has to be noted that when performed under identical conditions, this reaction is nearly quantitative under microwave heating whereas almost no outcome was observed under conventional conditions.