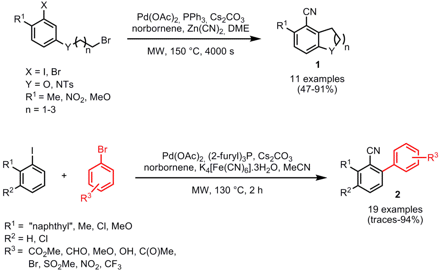
The group of Mark Lautens from the University of Toronto has reported on the Pd-catalyzed C-H functionalization reaction for the synthesis of highly substituted benzonitriles (J. PMID:27017949 Am. 1-Phenylbuta-2,3-dien-1-one Purity Chem. 4,6-Dichloro-5-nitropicolinic acid web Soc. 2007, 129, 15372. DOI: 10.1021/ja075599i). Bicyclic products 1 were obtained in one step by an intramolecular alkylation/cyanation sequence. Both electron-withdrawing and -donating o-substituents are tolerated. Tricyclic benzonitriles can be obtained via a double o-alkylation/cyanation sequence. Biaryl compounds 2 were synthesized via an intermolecular o-arylation/cyanation sequence employing electron-deficient aryl bromides. The reaction conditions regarding ligand, cyanation reagent and solvent had to be changed and when aryl iodides lacking an o-blocking group were employed, only complex mixtures of products were received. Important for both strategies is norbornene which is able to initiate the competitive C-H functionalization pathway, so that C-C and C-CN bond formation can occur sequentially.
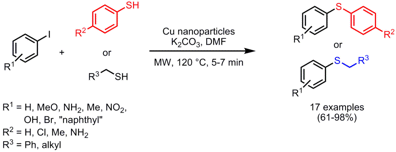
Cu Nanoparticle-Catalyzed Aryl-Sulfur Bond Formation
Brindaban Ranu and co-workers from the Indian Association for the Cultivation of Science in Kolkata have developed the ligand-free coupling of aryl iodides with thiophenols and alkanethiols under Cu nanoparticle (4-6 nm) catalysis (Adv. Synth. Catal. 2007, 349, 2690. DOI: 10.1002/adsc.200700289). The arylsulfide synthesis proceeded in good to excellent yields and high purities. Compared to conventional heating at 120 °C, the reaction times could be accelerated from 12-15 h to only 5-7 min. Other Cu sources such as metallic Cu, CuI or Cu powder lead to lower product yields, indicating the key role of Cu nanoparticles in this C-S coupling reaction.
Intramolecular Cycloaddition of Azomethin Ylides
In course of the preparation of a polycyclic pyrrolidine library, Mark Kurth and co-workers from the University of California, Davis, have disclosed an intramolecular azomethin ylide1,3-dipolar cycloaddition as key transformation (Org. Lett. 2007, 9, 5055. DOI: 10.1021/ol702301n). Fused pyrrolidine scaffolds 4 were obtained in high regio- and stereoselectivity via condensation of benzimidazole-carbaldehydes 1 with a secondary amino ester 2 to form the S-shaped ylide 3 and the subsequent intramolecular 1,3-dipolar cycloaddition of 3. Importantly, the nature of the secondary amine plays a crucial role in the outcome of the reaction (R1 = H, R2 = Ph: 10%; R1 = H, R2 = Me: 59%).
