A key step in the Gin synthesis of the hetesine alkaloid nominine reviewed
last week (
2007, August 6) was an intramolecular
Diels-Alder reaction. There have
been several other recent applications of intramolecular Diels-Alder
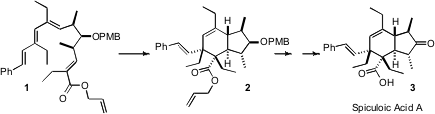
cyclizations in natural product synthesis. In the route to spiculoic acid A (3)
reported (Chem. 346704-04-9 supplier Commun. 2006, 2863.
DOI: 10.1039/b607035c)
by Victor Lee and Jack E.
Baldwin of Oxford University, the activating electron-withdrawing ester is
located at the end of the nonatriene 1. Under such circumstances,
cyclization is rapid at room temperature, even in this highly substituted
example, and it leads to the trans ring fusion, as illustrated.
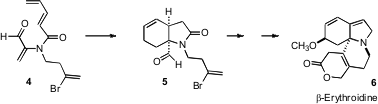
Retrosynthetic analysis of β-erythroidine (6) suggested (Org. Lett.
2006, 8,
3689.
DOI: 10.1021/ol061267r)
to Raymond L. Funk of Pennsylvania State University the intramolecular
Diels-Alder cyclization of the triene 4. 352525-25-8 manufacturer Note that in this case, the activating
carbonyl is internal on the nonatriene. PMID:24605203 Under such circumstances, the
cyclization requires heating, and leads to the cis ring fusion. The contrast
between the cyclization of 1 and the cyclization of 4 has been rationalized by
charge transfer in this concerted but non-synchronous reaction proceeding to
give initially the five-membered ring with nonatrienes such as 1, but initially
the nine-membered ring with nonatrienes such as 4.
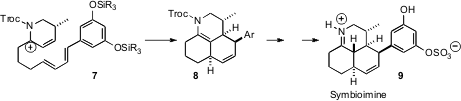
Allylic carbocations can also be effective dienophiles, as illustrated by the
cyclization of 7 to 8 in the course of the synthesis of symbioimine
9 developed
(Org. Lett. 2006, 8, 5605.
DOI: 10.1021/ol062333s)
by Barry B. Snider of Brandeis University. A very
different route to symbioimine was described (Angew. Chem. Int. Ed. 2006,
45,
4767.
DOI: 10.1002/anie.200601418)
by Martin E. Maier of the Universität Tübingen, based on the
intramolecular Diels-Alder cyclization of a decatriene.
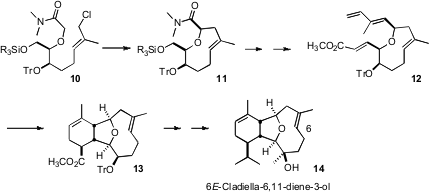
Syntheses of 6Z-cladiellin diterpenes, but not of 6E-cladiellins, had been
reported. The key to the intramolecular Diels-Alder approach to the E cladiellin
diterpenes devised (J. Am. Chem. Soc. 2006, 128, 15851.
DOI: 10.1021/ja065782w)
by Deukjoon Kim of Seoul
National University was the intramolecular alkylation that set the relative
configuration of 11.
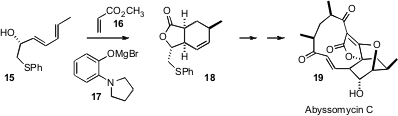
The dienophile and the diene do not have to be covalently attached for a
Diels-Alder reaction to be intramolecular. In the course of preparing starting
material for the synthesis of abyssomycin C (19), K. C. Nicolaou of Scripps, La
Jolla found (Angew. Chem. Int. Ed. 2006, 45, 3256.
DOI: 10.1002/anie.200601116)
that addition of methyl
acrylate 16 to the sorbic acid-derived alcohol 15 proceeded well only in the
presence of the magnesium salt 17, which is presumably serving to complex the
diene and the dienophile prior to cycloaddition.
Several other noteworthy advances in intramolecular Diels-Alder cyclization
are reported in Tetrahedron Lett. 2006, 47, 4671,
DOI: 10.1016/j.tetlet.2006.04.129;
Chem. Commun. 2006, 3646,
DOI: 10.1039/b605368h; J.
Org. Chem. 2006, 71, 6701,
DOI: 10.1021/jo061119e, and Org. Lett.
2006, 8, 5693,
DOI: 10.1021/ol062067i. The work described in
Chemistry Lett. 1989, 1947,
DOI: 10.1246/cl.1989.1947 is also worth re-reading.