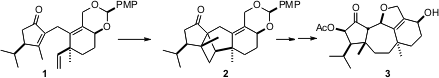
The guanacastepenes, of which guanacastepene E (3) is representative, initially elicited excitement because of their activity against drug resistant bacterial strains. Although the systemic toxicity of the guanacastepenes has cooled that enthusiasm, the guanacastepenes remain as architectural challenges. In particular, a convergent synthesis plan requires the development of a strategy for constructing the central 7-membered ring with control of relative and absolute configuration, particularly of the two angularly methylated quaternary centers. Erik J. PMID:36014399 Sorensen of Princeton University (J. Am. 6-Bromohexanenitrile uses Chem. Soc. 2006, 128, 7025.DOI: 10.1021/ja060847g)solved this problem by the intramolecular photochemical dimerization of 1, which delivered 2 with high diastereocontrol. (3-(4-Hydroxyphenyl)acryloyl)glycine Formula Directed ring fragmentation then led to 3.
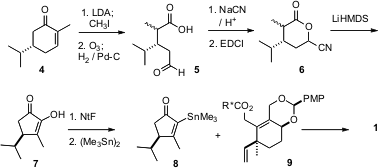
The enantiomercially-purecyclopentenone of 1 was prepared from the chiral pool starting material dihydrocarvone (4). Methylation followed by ozonolytic cleavage gave the aldehyde acid 5, which was converted into the cyanohydrin lactone 6. Intramolecular acylation of the derived nitrile anion proceeded smoothly, to give, after fragmentation, the 1,2-diketone7. Stannylation of the nonaflate led to 8, which was coupled with enantiomerically-pure 9 to give 1.
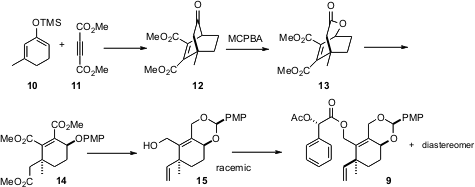
Construction of the cyclohexene 9 began with the Diels-Alder cycloaddition of the alkyne 11 to the diene 10, followed byBaeyer-Villiger cleavage to give 13. This established the quaternary center, and at the same time set the relative configuration of the secondary alcohol of 9, and thus of 3. The alcohol 15 so prepared was racemic. Screening of esters led to the mandelate acetate 9, the diastereomers of which could be separated by open column chromatography. The mandelate ester also proved to be an effective leaving group for the Pd-mediated coupling of 8 and 9 to give 1.
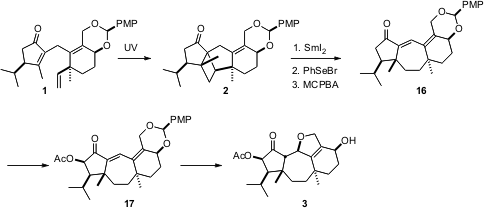
As anticipated, the intramolecular 2+2 photocyclization was directed by the adjacent isopropyl group, to give 2 with high diastereocontrol. While opening of carbonyl-activated fused cyclopropanes is amply precedented, far less was known about the opening of carbonyl-activated fused cyclobutanes. Happily, double one-electron reduction with excessSmI2 opened the desired bond, to give, after selenation and oxidation, the dienenone 16. The acetoxy group adjacent to the ketone was introduced by Rubottom oxidation. On deprotection of 17, the released primary alcohol spontaneously added to the enone, to give (-)-guanacastepene E (3) .