Synthesis: Part One of Three
We last reviewed organic synthesis applications of the Grubbs reaction on
April 19, 2004. The (relatively) robust nature of the commercially-available
catalyst and its commercial availability have spurred the expanding exploration
of the scope of this reaction. 790667-43-5 In stock Through this month, we will feature some recent
highlights. PMID:23849184 (Part Two /
Part Three)
The most straightforward application of the Grubbs reaction is to effect
homologation of a terminal vinyl group. One concern with the use of the second
generation Grubbs catalyst
3 is the cost, about $100/mmol. In conjunction with a total synthesis of
the macrolide RK-397, Frank McDonald of Emory reported (J. Am. G0-C14 Purity Chem. Soc.
2004, 126, 2495.
DOI: 10.1021/ja039618+)
that the conversion of
1 to 2 required 10 mol % of 3, but that it proceeded
efficiently with just
2 mol % of the Hoveyda Ru catalyst 4 (J. Am. Chem. Soc.
2000,
122. 8168.
DOI: 10.1021/ja001179g)
. The alkene so prepared was cleanly trans. An advantage of 4 is
that it avoids the use of the expensive PCy3. We will refer to 3 as
G2 and to 4 as H2.
Because of the functional group tolerance of the Grubbs catalyst 3, it
will operate even with complex substrates. In the course of a total synthesis of
(-)-cytisine, Giordano Lesma and Alessandra Silvani of the University of Milan
reported (Org. Lett. 2004, 6, 493.
DOI: 10.1021/ol0361507)
that
5 could be cyclized efficiently to 6.
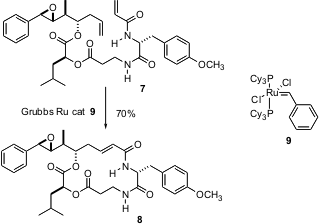
The even more spectacular cyclization of 7 to Arenastatin A 8
was reported (Tetrahedron Lett. 2004, 45, 5309.
DOI: 10.1016/j.tetlet.2004.04.164)
by Gunda Georg of the University of Kansas. In this case, the catalyst
used was the first generation Grubbs catalyst,
9.