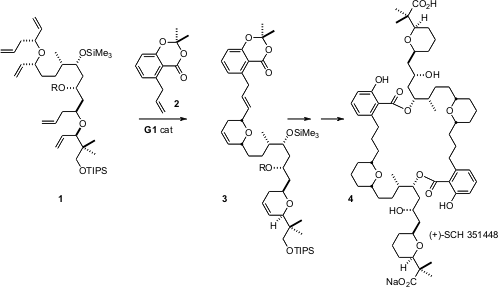
The symmetrical macrodiolide (+)-SCH 351448 (4) is the only known selective activator of transcription from the low density lipoprotein receptor. The highly convergent synthesis of 4 from 1 and 2 reported (Org. 6-Chloropyridazine-3-carbaldehyde Order Lett. 2006, 8, 2887. DOI: 10.1021/ol061073b)by Michael T. Crimmins of the University of North Carolina illustrates the power of the chiral glycidyl anion approach for the preparation of α,α’-bis chiral ethers. PMID:29844565 2055840-60-1 site
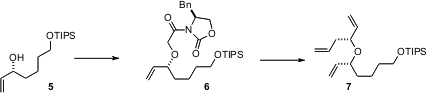
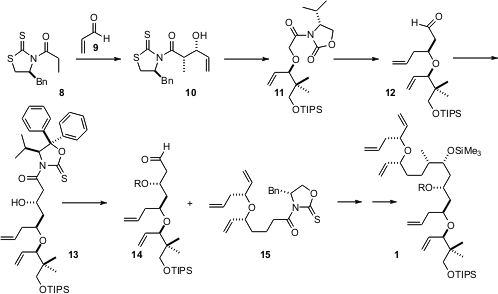
The upper half of 1 was assembled by Sharpless resolution of the alcohol5. Allylation of the enolate derived from 6 then delivered, after reduction and methylenation, the triene 7. The lower half of 1 was prepared by condensation of the anion derived from 8 with acrolein 9. Methylation of product10 led to 11, which was again allylated, to give, after homologation, the aldehyde12. Condensation with a chiral acetate equivalent gave 13. The corresponding aldehyde 14 was then added to the enolate of 15, derived from 7, to arrive at 1.
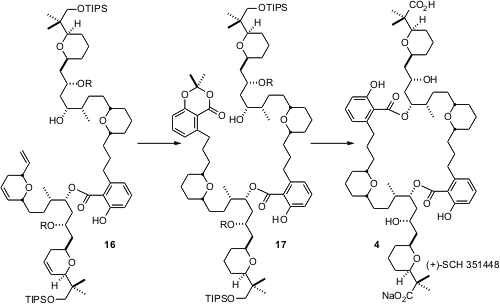
Although the tandemring closing metathesis – homologation of 1 with2 proceeded spectacularly well, to give 3 in 88% yield, simple dimerization of 3 did not deliver the desired 4. As an alternative, 15 was cyclized with G1, then acylated with reduced 3 to give 16. Intramolecular acylation of 17 then worked well, leading to 4.