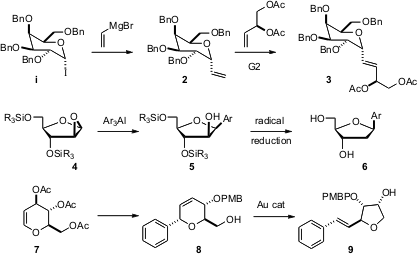
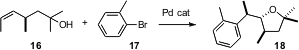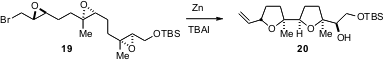Sugars are still useful chiral pool starting materials for the preparation of cyclic ethers. Jacquelyn Gervay-Hague of the University of California, Davis, has extended (Org Lett. 1047991-79-6 web 2006, 8, 5765. DOI: 10.1021/ol062354m)her β-galactosyl iodide work, showing that 1 couples with vinyl magnesium bromide to give 2 with high diastereocontrol. 1-Methyl-1H-indazol-5-ol manufacturer The alkene 2 is easily homologated to the C-glycoside 3. Oliver Seitz of the Humboldt-Universität, Berlin, has developed (Org Lett. 2006, 8, 4319. PMID:35850484 DOI: 10.1021/ol061701p)a simple three-step preparation of the epoxide 4 from thymidine. Opening of 4 with Ar3Al delivered the β-aryl-C-nucleoside 5 with high diastereocontrol. Free radical deoxygenation then gave 6. John A. Porco, Jr. and Scott E. Schaus of Boston University have found (Org Lett. 2006, 8, 5065. DOI: 10.1021/ol0618252)a route from the inexpensive glucal 7 to the enantiomerically-puretetrahydrofuran 9. The Au-catalyzed rearrangement of 8 to 9 proceeds with 10:1 diastereocontrol.
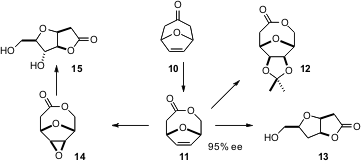
It is also possible to expand the chiral pool with enantioselective catalysis. Marko D. Mihovilovic of the Vienna University of Technology has been working (Chem. Comm. 2006, 3214. DOI: 10.1039/b606633j)toward making biocatalysis more practical by employing enzymes, in this case cyclopentanone monooxygenase, over-expressed in whole cell systems. Using this approach, it is not necessary to add expensive co-factors. In a bioreactor, he was able to carry out the enantioselectiveBaeyer-Villiger oxidation of 10 on a multi-gram scale. The lactone 11 was readily carried on to 12 – 15.
John P. Wolfe of the University of Michigan has been exploring (J. Am. Chem. Soc. 2006, 128, 2893, DOI: 10.1021/ja057489m;Tetrahedron Lett. 2006, 47, 2793, DOI: 10.1016/j.tetlet.2006.02.066)Pd-catalyzed diastereoselective tetrahydrofuran formation. Remarkably, the single stereogenic center of 16 directed the cyclization, leading to predominant (86:9:5) formation of 18.
The bis-tetrahydrofuran 20 is the core of the antibiotic ionomycin. James A. Marshall of the University of Virginia has shown (Org. Lett. 2006, 8, 4375. DOI: 10.1021/ol061826u)that the enantiomerically-pure tris epoxide 19, readily prepared from farnesol, underwent smoooth reductive cyclization to 20.
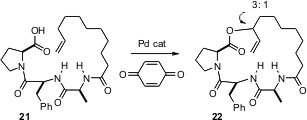
Macrolactone (“macrolide”) natural products are widely distributed. Both C-O and C-C bond forming strategies have been used for the preparation of macrolides. In either case, two functionalized carbon atoms were being connected. M. Christina White of the University of Illinois has now reported (J. Am. Chem. Soc. 2006, 128, 9032. DOI: 10.1021/ja063096r)what appears to be a general route to macrolides using C-H activation, as represented by the conversion of 21 to 22. It is impressive that a particular C-H bond can be specifically replaced with a C-O bond. It is even more impressive that the reaction proceeded with substantial diastereocontrol.